FIGURE 1.
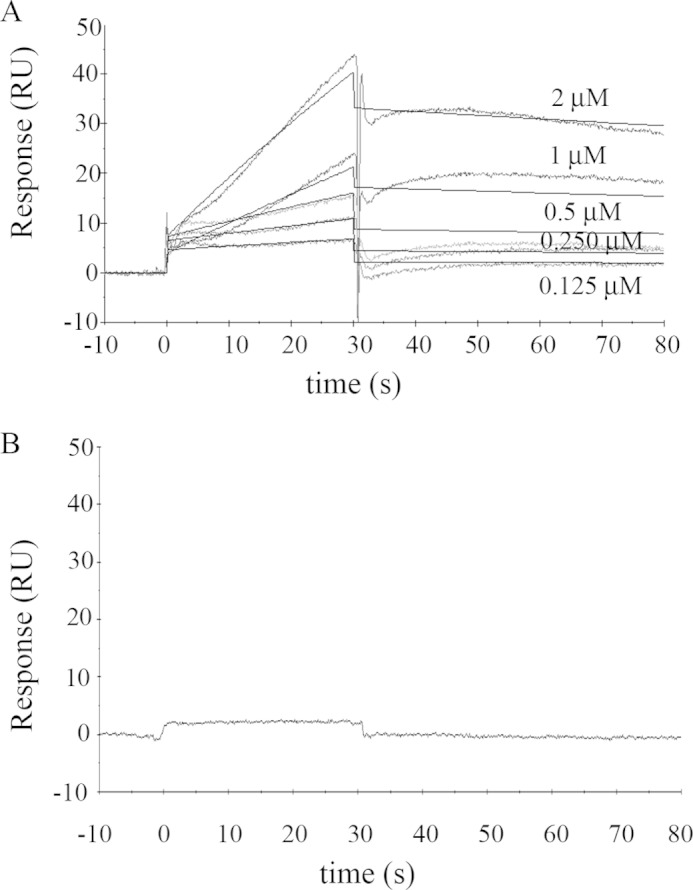
Binding of solubilized human erythrocyte PMCA to immobilized G-actin. A, representative sensorgram of PMCA binding to immobilized G-actin obtained for different PMCA concentrations in the range of 0.125 to 2 μm. G-actin immobilization level was ∼ 800 RU. The running buffer consisted of a low ionic strength Tris-HCl buffer with 70 μm Ca2+ and 0.005% C12E10 to maintain PMCA solubilized in micelles. The time for the association phase was set to 30 s. Steady state for binding interaction was not attained, and therefore a kinetic analysis was carried out. Curves were corrected for bulk effects by simple subtraction of the corresponding control sensorgrams. The dark gray lines represent the experimental curves, and the continuous black lines are the corresponding fits. B, representative sensorgram of PMCA (0.75 μm) injected in a running buffer devoid of Ca2+ by the addition of 2 mm EGTA shows no binding interaction. All other running conditions were identical to those in A. Note that human erythrocyte PMCA corresponds mostly to isoform PMCA4b (see under “Materials and Methods”).
