Figure 4.
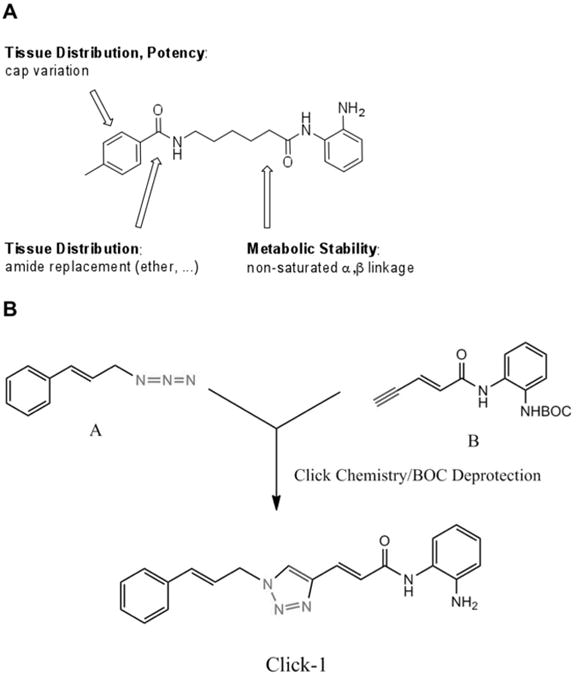
Compounds with improved pharmacological properties. (A) Brain penetration can be improved by elimination of the left amide, and replacement with an ether, olefin, or ketone. Metabolic stability can be improved by introducing a non-saturated α/β linkage adjacent to the right amide, which prevents formation of a benzimidazole. (B) Synthetic route to compounds with these replacements using Cu(I)-catalyzed click chemistry, where azide A is reacted with alkyne B, and after Boc deprotection, a triazole is generated in the linker region of the histone deacetylase inhibitor.59
