Fig. 3.
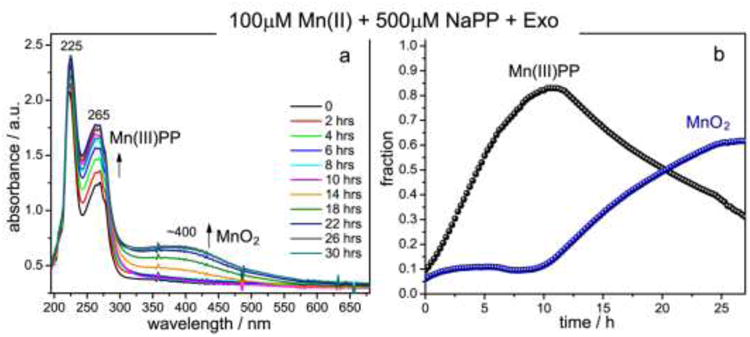
Time-resolved UV-vis absorption spectral measurements of Mn(II) oxidation by exosporium at room temperature. (a) Selected UV-vis absorption spectra of the reaction mixture taken at the indicated time points during the course of Mn(II) oxidation. The growth of 265 nm band indicates formation of the Mn(III) intermediate trapped by pyrophosphate; the 400 nm band is due to formation of particulate Mn oxides. (b) Time courses followed by Mn(III)-pyrophosphate and MnO2 species during the 30 h UV-vis absorption experiment and obtained from the fit of the absorption spectrum at each time point to a linear combination of the component spectra depicted in Fig.2
