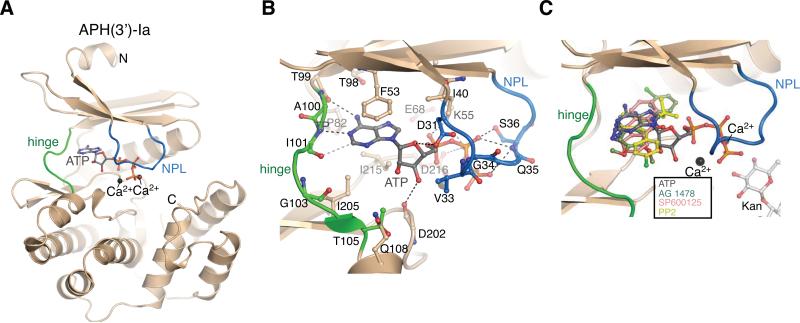
Figure 1. Overview of APH(3’)-Ia structures.
A) Structure of the APH(3’)-Ia•Ca2+•ATP complex. APH(3’)-Ia hinge region is coloured green, nucleotide-positioning loop is coloured blue, Ca2+ ions shown in black spheres. N- and C-termini are labeled. B) Interactions between APH(3’)-Ia and ATP. C) Superposition of APH(3’)-Ia•ATP, APH(3’)-Ia•kanamycin•SP600125, APH(3’)-Ia•kanamycin•PP2 (representative of APH(3’)-Ia•kanamycin•PP1), APH(3’)-Ia•kanamycin•AG 1478 complexes. As hinge region of APH(3’)-Ia is nearly identical across all inhibitor complexes, only one copy of the enzyme is shown.