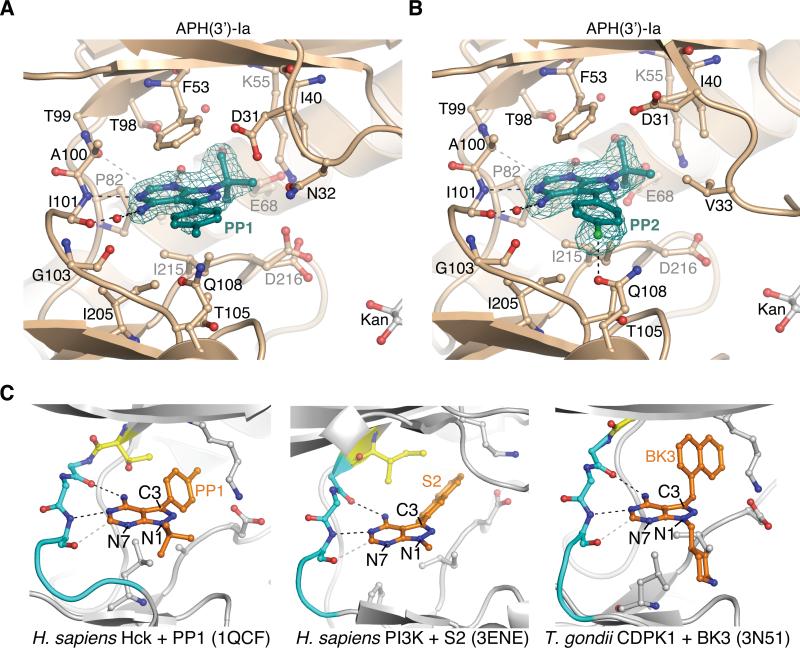
Figure 4.
Binding mode of PP-based compounds to APH(3’)-Ia and comparison with PP-based compounds bound to ePKs
A) Structure of APH(3’)-Ia•kanamycin•PP1 complex. B) Structure of APH(3’)-Ia•kanamycin•PP2 complex. Electron density shown for both inhibitors is simulated annealing Fo-Fc omit density at 3.0 σ. C) Binding modes of PP-based inhibitors to ePKs. Left = PP1 bound to H. sapiens Hck (PDB accession 1QCF); middle = S2 bound to H. sapiens PI3K (PDB accession 3ENE); right = BK3 bound to T. gondii CDPK1 (PDB accession 3N51). Gatekeeper residues are coloured yellow, hinge residues cyan, also shown are sticks of conserved Lys-Asp/Glu salt bridge in active site.