Abstract
Adolescence is a developmental period when many teenagers first drink alcohol and often engage in binge drinking. Early onset of alcohol is linked to increased risk of stress-related disorders in adulthood in humans, suggesting that alcohol may interfere with development of the stress regulatory system. We investigated the effect of voluntary alcohol exposure on corticotropin-releasing factor (CRF) peptide producing cells in the central nucleus of the amygdala (CeA) in adolescent male and female rats. These cells are important for the autonomic and behavioral responses to stress, have been implicated in addiction, and change over adolescent development. Animals self-administered sweetened alcohol during early adolescence (postnatal days 28-42) and brains were obtained on postnatal day 43 for CRF peptide immunolabeling. Females had fewer CRF immunoreactive (-ir) cells in the CeA compared to males. In both males and females, alcohol self-administration reduced the number of CRF-ir cells in the CeA compared to control conditions in which rats self-administered equivalent levels of sweetened water that did not contain alcohol. Reduced peptide labeling was not observed in the bed nucleus of the stria terminalis (BNST), indicating regional specificity of these changes. Alterations within the CRF cell population of the amygdala may have important implications for susceptibility to alcohol and stress disorders during adolescence and later on in life.
Introduction
Adolescence could be considered a “perfect storm” for mental health vulnerability. This is a developmental period when both humans and rodents increasingly seek out new sensations and risks, including binge drinking (Reinherz et al., 1993; Spear, 2000). This behavior is thought to be a coping mechanism for stress that can contribute to the development of certain psychopathologies (Compas et al., 1993; Spear, 2000). Consuming alcohol early in adolescence is highly predictive of alcoholism vulnerability later in adulthood (Chou and Pickering, 1992; Grant and Dawson, 1997; DeWit et al., 2000; Zeigler et al., 2005; Johnston et al., 2007; Dawson et al., 2008; Donovan and Molina, 2008). It is possible that adolescent alcohol exposure impacts the development of stress regulatory systems in the brain to alter mental health risk in adulthood.
Corticotropin-releasing factor (CRF, also known as corticotropin-releasing hormone or CRH) is a 41-amino acid peptide that mediates the autonomic, behavioral, and neuroendocrine responses to stress (Vale et al., 1981; Bale and Vale, 2004). Studies in adult rodents indicate that CRF in the central nucleus of the amygdala (CeA) is sensitive to alcohol and neuroadaptive changes to these peptide cells and their receptors are thought to contribute to the emotional and behavioral symptoms of addiction (Koob, 2003, 2008; Heilig and Koob, 2007; Gilpin and Roberto, 2012). Similarly, CRF in the bed nucleus of the stria terminalis (BNST), which bi-directionally communicates with the CeA (Dong et al., 2001a,b; Dong and Swanson, 2003, 2004), is also implicated in alcohol withdrawal (Olive et al., 2002) and relapse (Le et al., 2000; Marinelli et al., 2007).
The present study explored the effects of alcohol exposure during early adolescence on CRF peptide-expressing cells in the CeA and BNST of male and female rats. We used a preclinical model that captures some of the characteristics of teenage drinking (voluntary intake of sweetened alcoholic beverages and intermittent access), and leads to augmented relapse-like drinking in adulthood in rats (Gilpin et al., 2012). The current study had three main objectives. First, we tested the hypothesis that alcohol impacts CRF peptide-expressing cells during adolescence. We previously found that adult male rats with a history of voluntary adolescent binge drinking and prolonged drinking in adulthood had a reduction in CRF-immunoreactive (-ir) cells in the CeA (Gilpin et al., 2012), similar to what has been observed in alcohol-withdrawn dependent rats (Zorrilla et al., 2001; Funk et al., 2006). Because of the extensive drinking history in adulthood, it was not known whether changes in the CeA CRF system took place early in adolescence or alternatively emerged as the animals aged and/or had extended exposure to drinking in adulthood. A second objective was to determine whether these changes extended to another addiction-related population of neurons, CRF cells within the BNST. Third, we tested the hypothesis that adolescent alcohol drinking has a more robust effect on CRF in females. Clinically, alcohol abuse has increased steadily over the past few decades in women (Bradley et al., 1998). Moreover, women show the first symptoms of alcohol-related problems sooner after the first drinking experience compared to men (Bradley et al., 1998). We reasoned that CRF stress peptide cells would be more sensitive to adolescent binge drinking in female rats because they have higher stress hormone levels (adrenocorticotropic hormone or ACTH and corticosterone) before and after stress (e.g., Le Mevel et al., 1979; Jezova et al., 1996; Richardson et al., 2006), and in response to alcohol delivered directly to the brain (e.g., Larkin et al., 2010).
Experimental Procedures
Animals
Twenty-two adolescent Wistar rats (11 males and 11 females) obtained from Charles River (Wilmington, MA, USA) were used in this study. Animals arrived on postnatal day (PD) 18 and were weaned on PD 21. They were housed in groups of three in plastic cages with wood chip bedding under a 12-h normal light cycle (lights on at 8 AM). All procedures met the guidelines of the University of Massachusetts Institutional Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Operant training (PD 25-27)
The timeline of the experiment is shown in Fig 1. All animals were first trained during their dark cycle to self-administer sweetened water with methods similar to the model we described previously (Gilpin et al., 2012). Animals were trained in operant boxes individually housed within sound-attenuating ventilated cubicles to minimize environmental disturbances (Med Associates Inc., VT, USA). The operant boxes had two retractable levers located 4 cm above a grid floor and 4.5 cm to either side of a 2-well aluminum drinking cup (custom made by Behavioral Pharma, San Diego, CA, USA). A single lever-press activated a cue light, and an infusion syringe pump that delivered 0.1 ml of fluid to the appropriate well over a period of 0.5 s. Lever presses that occurred during the 0.5 s of pump activation were not recorded and did not result in fluid delivery. Operant responses were recorded by custom software running on a PC computer.
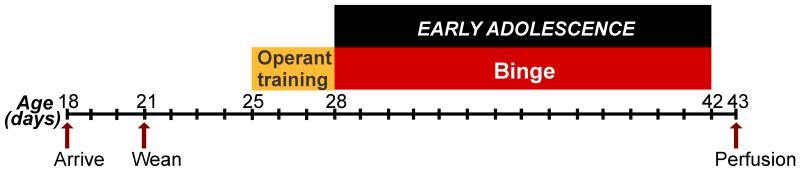
Figure 1. Timeline of treatment and brain collection for voluntary binge drinking experiments in adolescent male and female Wistar rats.
Voluntary binge alcohol self-administration took place during early adolescence (postnatal days 28-42) and brains were collected after the last binge session.
Rats were initially trained in pairs or triads for a few sessions because we have found in our laboratory that this facilitates training. A single lever was continuously available and presses resulted in delivery of 0.1 ml sweetened solution (3% glucose, 0.125% saccharin in tap water) on a fixed-ratio 1 (FR1) schedule. Once the pairs or triads established lever responding, they were individually placed in operant boxes. All animals were trained on a “binge” schedule with sweetened water at least 1-2 days before voluntary binge self-administration alcohol treatment began on PD 28. For binge training, animals were allowed access to a single lever (FR1 schedule, rewarded with sweetened water only) in six 30-min sessions at 60-min intervals in an overnight session. Food was available ad libitum during the training and binge drinking sessions of this study. Operant behavior under this schedule was used to split the animals into “control” and “binge” groups that were balanced for self-administration behavior. Occasionally animals do not learn how to lever press during the short training period, but untrained animals are always excluded from our experiments prior to dividing animals into the two treatment groups. In the present study, one female rat had not successfully trained and was eliminated from the study prior to dividing into the treatment groups. We usually assign more animals to binge groups in our studies to account for individual variability in exposure to alcohol. Therefore, the group sizes for the behavioral portion of the study were: 8 control rats (4 males, 4 females); 13 binge rats (7 males, 6 females). One binge female rat was lost from the BNST CRF portion of the study because of tissue loss during the immunolabeling processing.
Voluntary binge drinking (PD 28-42)
At the start of early adolescence (PD 28), animals were exposed to six 30-min sessions at 60-min intervals during the animals’ dark cycle for two consecutive weeks. In each session, animals could press a lever that delivered 0.1 ml sweetened alcohol (3% glucose, 0.125% saccharin, 8% w/v alcohol, which is 10% v/v alcohol) for binge animals and sweetened water (3% glucose, 0.125% saccharin) for control animals on a FR1 schedule. A PC computer connected to the operant boxes was used to cap the maximum number of responses per 30 min bout to keep the level of self-administration in control groups close to the level of binge groups. After the maximum number of responses was reached, the lever retracted until the next scheduled self-administration session to avoid extinguishing self-administration responding in the control animals. With this design, alcohol binge and control animals had similar experiences with operant self-administration and glucose/saccharin intake, and the only difference between the two groups was exposure to alcohol. Water and food was available ad libitum during the binge sessions, ensuring that all alcohol drinking was purely voluntary and not motivated by thirst or hunger.
Perfusion and immunohistochemistry
On PD 43, male and female rats were deeply anesthetized using 35% chloral hydrate (2-4 ml/kg, i.p.), a dose that produced rapid sedation and minimal stress to the animal as evidenced by basal levels of stress-related transcripts (Lee et al., 1999). Animals were then intracardially perfused with 0.9% saline followed by 4% paraformaldehyde/0.1 M borate buffer, pH 9.5. Brains were post-fixed for 4 h in the same fixative and then placed in 20% sucrose solution for 24-48 h in 4 °C. Brains were snap frozen using −50°C isopentane (2-methylbutane, Fisher Scientific, Pittsburgh, PA, USA) and stored in −80 °C. Coronal brain tissue sections (35-μm thick) were cut on a freezing microtome and stored at −20 °C in cryoprotectant (50% 0.1 M phosphate-buffered saline, 30% ethylene glycol, 30% sucrose and 1% polyvinyl pyrrolidone) until immunohistochemistry. Free-floating 3,3′-diaminobezidine (DAB, Vector Laboratories, Burlingame, CA, USA) immunohistochemistry procedures followed the protocol described previously (Gilpin et al., 2012) using a primary rabbit anti-h/rCRF antiserum solution (1:5000, generously provided by Dr. Wylie Vale, Salk Institute) and a goat anti-rabbit secondary antiserum solution (1:200). After immunolabeling, sections were mounted on glass slides and coverslipped for microscopic analysis.
CRF cell count analysis
Cells containing CRF peptide were counted by an experimenter blind to the treatment of the animal using a Leica microscope at 400× magnification (40× objective and 10× eyepiece) and a standard thumb-operated tally counter. Cells containing labeling throughout the soma and neuronal processes, with a clear border around the soma were considered CRF-ir and counted as previously described. Adjusting the optical axis (z-axis) of the microscope allowed for clearer visualization of the depth of the soma to better distinguish the soma from a CRF-ir cell versus any ambiguous particle on the section. Also, adjusting the z-axis allowed for clearer visualization of individual cell borders when cells were clustered together (Gilpin et al., 2012).
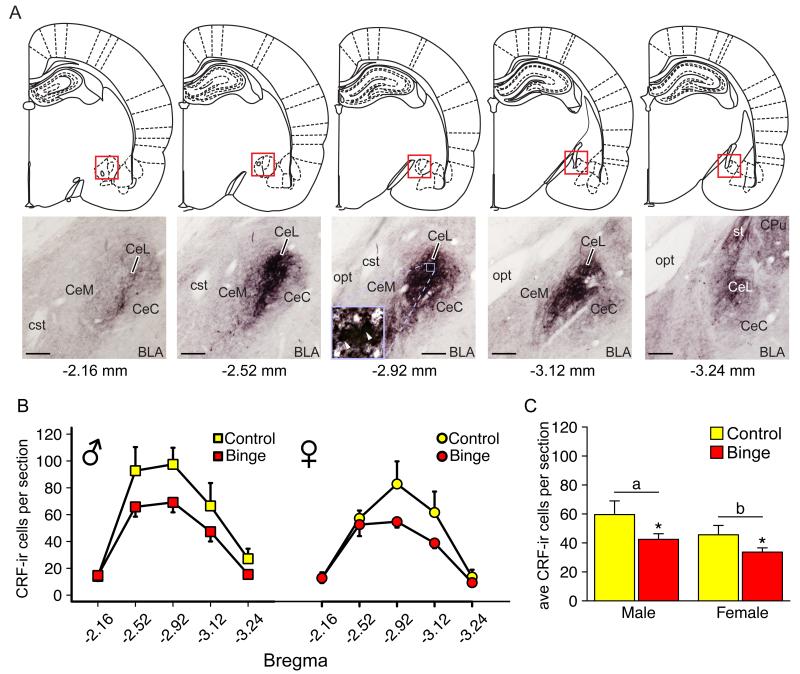
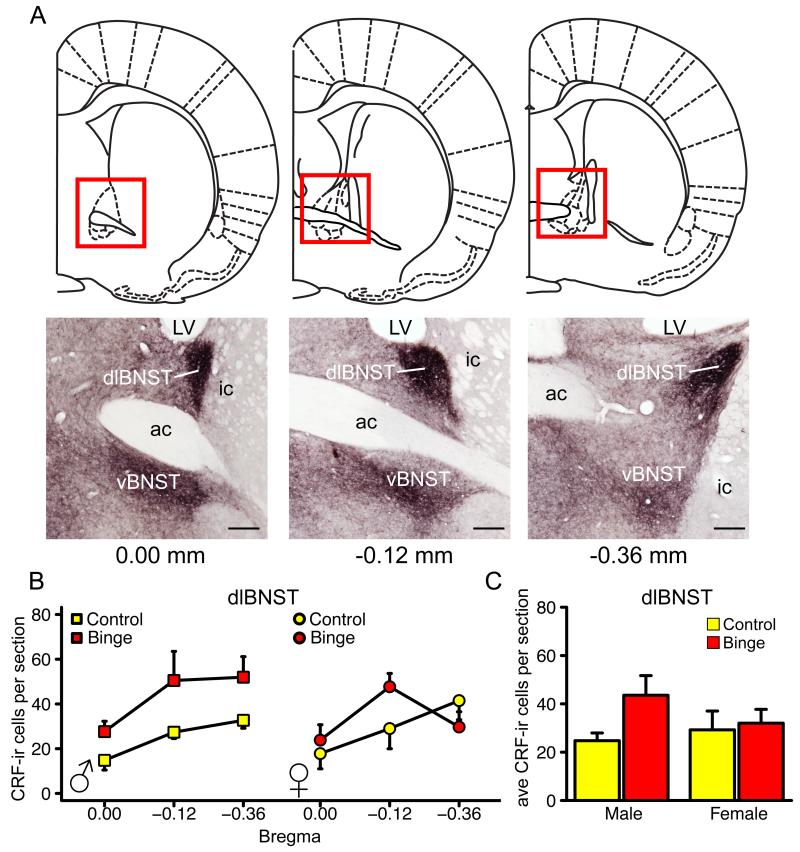
Fig 3A illustrates the coronal sections analyzed for CRF-ir cell counts. Every 5th section throughout the BNST and CeA was used for CRF labeling and analysis. CRF-ir cells were analyzed at the following anatomical locations, relative to Bregma, for the CeA: −2.16 mm, −2.52 mm, −2.92 mm, −3.12 mm and −3.24 mm. CRF-ir cells were analyzed at the following anatomical locations, relative to Bregma, for the BNST: 0.00 mm, −0.12 mm, and −0.36 mm. These anatomical locations were determined according to the Rat Brain in Stereotaxic Coordinates Atlas (Paxinos and Watson, 2007).
Statistical analysis
Body weight was analyzed using mixed-model ANOVAs with age as a within-subject variable and binge treatment as a between-subject variable for males and females. Cumulative alcohol intake (g/kg) over the two-week treatment period was analyzed in binge animals using a mixed-model ANOVA with sex as a between-subject variable and PD as a within-subject variable. Cumulative glucose intake (g/kg) over the two-week treatment period was analyzed in all animals using a mixed model ANOVA with sex and binge treatment as between-subject variables and PD as a within-subject variable. CRF-ir cells per section (averaged across all sampled sections from a specific brain region) were analyzed for the BNST and CeA CRF populations using 2-way ANOVAs with sex and binge treatment as between-subject variables. Differences were considered significant when p ≤ 0.05. Wherever appropriate, data are expressed as mean ± SEM. All statistical analyses were conducted using the R statistical software package (R Development Core Team, 2012).
Results
Alcohol drinking in adolescent male and female rats
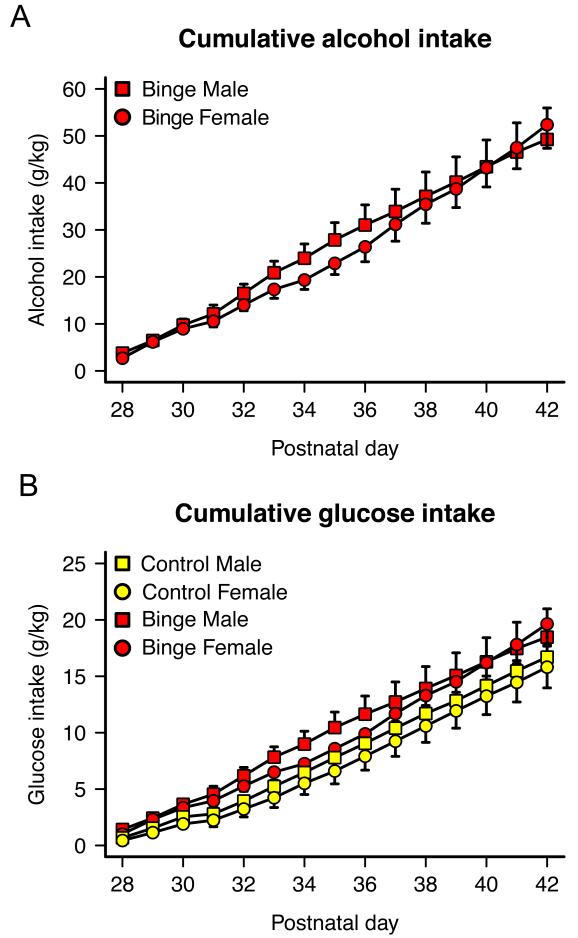
Operant self-administration data are shown in Fig 2. Fig 2A shows cumulative alcohol intake (g/kg) in the male and female alcohol groups over the treatment period. Animals consumed 18.5–62.4 g/kg alcohol intake between PDs 28 and 42. Fig. 2B shows cumulative glucose intake (g/kg) over the treatment period (one of the two sweeteners in the rewarding solutions). Glucose intake was calculated based on the proportion of glucose (3%) in the sweetened alcohol consumed by binge animals or sweetened water consumed by control animals. There was a trend of cumulative glucose intake being slightly higher in binge rats during the two-week period of exposure compared to control rats but this difference was not statistically significant (F(1,17) = 4.04, p = 0.061). There were no significant sex differences in cumulative alcohol intake or cumulative sweetened water intake during the two-week period (all ps > 0.05).
Figure 2. Overview of alcohol and glucose intake during the two weeks of self-administration in adolescent male and female Wistar rats.
(A) Cumulative alcohol intake (g/kg) for binge rats, and (B) cumulative glucose intake (g/kg) for binge and control rats over the treatment period. Total alcohol and glucose intake was not significantly different across groups (all ps > 0.05).
Effect of alcohol on CRF peptide expressing cells in the CeA and BNST
Figure 3A shows an anatomical map describing the CeA sections used for analysis and CRF labeling within those sections. Figure 3B shows the distribution of CRF-ir cells across the five anatomical locations analyzed within the CeA. Decreases in CRF-ir cell number occurred mostly between −2.52 and −3.12 mm, relative to Bregma, where the majority of cells are located within the CeA. Figure 3C shows the number of CRF-ir cells per section averaged across the entire CeA. There was a main effect of treatment (F(1,17) = 9.29, *p = 0.007, Fig. 3C). Alcohol significantly reduced the average number of CRF-ir cells/section in the CeA in male and female rats. There was also a main effect of sex on the average number of CRF-ir cells per section in the CeA (F(1,17) = 5.35, *p = 0.033, Fig. 3C). Males had significantly more CRF-ir cells compared to females. There was not a significant interaction between sex and treatment, indicating that alcohol caused similar changes in CRF-ir cells in both sexes. There were no significant effects of alcohol or sex on adolescent male and female CRF-ir cells in the BNST (all ps > 0.05, Fig. 4). However, there was a trend of an increase in peptide-labeled cell number in the dlBNST; a direction opposite of that which was observed in the CeA.
Figure 3. Effect of binge drinking on CRF-ir cell number in the CeA of adolescent male and female Wistar rats.
(A) Brain sections between −2.16 mm to −3.24 mm, relative to Bregma, were sampled for CeA CRF-ir cell counting. Micrographs showed the CRF-ir population in sampled sections. CRF-ir cells are densely populated in lateral CeA. Individual cells were identified and counted by observing at different focal planes (inset). Scale bar, 200 μm. (B) Distribution of CRF-ir cells across the five anatomical locations analyzed. (C) Female rats have significantly fewer CRF-ir cells in the CeA compared to male rats. Voluntary alcohol drinking significantly reduced CRF-ir cell number in both sexes. Data are expressed as mean ± SEM (n = 4-7 rats for each sex and treatment group). *p < 0.05 indicates significant effect of sex, a,bp < 0.05 indicates significant main effect of treatment. Abbreviations: BLA, basolateral amydaloid nucleus; CeC, central amydaloid nucleus, capsular part; CeL, central amydaloid nucleus, lateral division; CeM, central amydaloid nucleus, medial division; CPu, caudate putamen; cst, commissure stria terminalis; opt, optic tract; st, stria terminalis.
Figure 4. Effect of binge drinking on CRF-ir cell number in the BNST of adolescent male and female Wistar rats.
(A) Brain sections between 0.00 mm to −0.36 mm, relative to Bregma, were sampled for BNST CRF-ir cell counting. Micrographs showed the CRF-ir population in sampled sections. Scale bar, 200 μm. Distribution of CRF-ir cells across the three anatomical locations in dlBNST (B) were analyzed. The BNST CRF-ir cell number was not significantly different among groups in either dlBNST (C) or vBNST (data not shown). Data are expressed as mean ± SEM (n = 4-6 rats for each sex and treatment group). Abbreviations: ac, anterior commissure; dlBNST, bed nucleus of the stria terminalis, dorsolateral division; ic, internal capsule; LV, lateral ventricle; vBNST, bed nucleus of the stria terminalis, ventral division.
Effect of alcohol on body weight
Daily body weights were taken to determine if alcohol significantly altered the physical development of the adolescent animals. We found no difference in body weight between the two treatment groups indicating that the differences in CRF-ir cell number are not dependent on physical growth (all ps > 0.05, Table 1).
Table 1. Mean (±SEM) body weight (in grams) on postnatal day 28, 36 and 42 in binge and control male and female Wistar rats.
| PD28 | PD36 | PD42 | |
|---|---|---|---|
| Control Males | 95 ± 3.1 | 158 ± 3.9 | 198 ± 3.4 |
| Binge Males | 97 ± 4.4 | 168 ± 4.7 | 218 ± 6.5 |
| Control Females | 96 ± 7.2 | 150 ± 9.8 | 181 ± 12.4 |
| Binge Females | 92 ± 5.6 | 144 ± 4.7 | 174 ± 4.2 |
Discussion
CRF is known to play a role in binge drinking and dependence in adulthood in humans (Treutlein et al., 2006; Blomeyer et al., 2008; Schmid et al., 2010), non-human primates (Barr et al., 2009), and rodents (e.g., Ciccocioppo et al., 2006; Funk et al., 2006; Hansson et al., 2006; Gehlert et al., 2007; Marinelli et al., 2007; Richardson et al., 2008b; Lowery et al., 2010). Drinking early in adolescence is linked to increased risk of alcoholism and other mental disorders later in adulthood (Chou and Pickering, 1992; Courtney and Polich, 2009), suggesting that drinking at this young age may alter development of the stress regulatory system. In support of this hypothesis, we previously found evidence of reduced CRF peptide-labeled cells in the CeA and higher relapse-like drinking in adulthood in male rats that drank early in adolescence and again throughout adulthood (Gilpin et al., 2012). Because this change was evident 1 month into abstinence, which was several months after the initial adolescent treatment had ended, it was reasonable to assume it emerged after prolonged exposure to drinking in adulthood. The present study took the critical first steps to establish when the change first emerged in this CRF population, and whether reduced peptide labeling was brain region-specific and sex-specific. Altogether our data indicate that adolescent alcohol (1) impacts this population of cells by the end of the two-week voluntary binge exposure early in adolescence (rather than a delayed or indirect effect of adult alcohol drinking and/or age), (2) the effect occurs in both males and females, and (3) CRF-ir cell number changes are brain region-specific and do not reflect a global decrease in CRF peptide expression, as CRF-ir cells were not reduced in the BNST. It will be important in future work to explore whether peptide changes within this specific population of stress regulatory cells functionally contributes to behavioral vulnerabilities later in life.
Overall, CRF peptide-expressing cells were fewer in number within the CeA of females. Alcohol caused a further 20% reduction in CRF-ir cells in females and a 33% reduction in males. Although this trend of a sex difference in the proportional decrease in cell number in binge rats was not significantly different, it suggests less—not more (as we had hypothesized)—sensitivity of female CeA CRF cells to alcohol. The fact that males and females consumed similar amounts of alcohol over the two-week treatment period (~50 g/kg) supports this interpretation. However, one possibility is that subtle differences in the rate at which males and females drank within the 30-min bouts or metabolism could occlude the detection of a sex difference in CRF sensitivity. We cannot presently rule out this possibility because our previous report detailing the pattern of intake and binge-like blood alcohol levels (≥0.08 g/dL, National Institute of Alcohol Abuse and Alcoholism, 2004) was done exclusively in males (Gilpin et al., 2012).
Alcohol self-administration may have caused a neuroadaptive change to CeA CRF cells over the two-week treatment period through direct and indirect pathways. Alcohol directly stimulates release and increases mRNA levels in CRF cells (Li et al., 2005). Alcohol self-administration has also been shown to activate the hypothalamic pituitary adrenal axis (HPA) in adult male rats (Richardson et al., 2008a), and high dose alcohol injections impact the developing HPA axis in adolescent animals (Przybycien-Szymanska et al., 2009). Corticosterone is known to positively feedback on CeA CRF cells (but not BNST cells) by inducing the transcription of CRF mRNA (reviewed in Watts, 2005). It is therefore conceivable that alcohol repeatedly activated CeA CRF cells by these different mechanisms, inducing modifications to this population that are evident by the exposure period (present study) and persist several months into adulthood long after alcohol exposure has ceased (Gilpin et al., 2012).
Developmental plasticity may also partially explain why alcohol differentially impacted CeA and BNST CRF cell populations. CeA CRF-ir cells undergo substantial change prior to and throughout adolescent development. Between PDs 10 and 45 CRF peptide-labeled cells double in number in the CeA—but not in the BNST—of male and female rats (Carty et al., 2010). CRF peptide immunoreactive density and cell counts in the CeA have been shown to decrease from mid-adolescence to adulthood in singly-housed male rats (Wills et al., 2010). The timing of the drinking in the present study suggests that alcohol may have interfered with the developmental increase in peptide that normally occurs in this CeA population of CRF neurons in early adolescence.
Decreased CRF-ir density has also been observed in the CeA of male rats that experienced repeated alcohol intoxication/withdrawal cycles by ethanol liquid diet exposure (Zorrilla et al., 2001; Wills et al., 2010) and intermittent alcohol vapor exposure (Funk et al., 2006), which is sufficient to produce physical dependence (Richardson et al., 2008a). In these studies, reduced peptide labeling is thought to reflect increased release because elevated extracellular CRF peptide levels within the CeA have been observed in adult dependent male rats after withdrawal from chronic alcohol (Merlo-Pich et al., 1995). Accordingly, females and alcohol-exposed animals in the current study may have fewer CRF-ir cells detected because of heightened activity of these cells (e.g., low peptide levels within the cell body due to release). Additional labeling methods such as in situ hybridization and the use of additional makers for co-localized proteins, in conjunction with in vivo tools such as microdialysis, could help to determine whether the peptide changes reflect active release from these cells or a true modification in cell number (cell death or alteration in phenotype). Given the substantial role these cells play in addiction vulnerability (Gilpin and Roberto, 2012), it will also be important to explore whether decreased CRF peptide expression functionally contributes to augmented relapse-like drinking observed in adult rats with a history of adolescent binge drinking or alcohol dependence (Gilpin et al., 2012).
Acknowledgments
This research was supported by a grant from the National Institutes of Health and National Institute on Alcohol Abuse and Alcoholism (AA021013) and by start-up funds from the University of Massachusetts Amherst. We thank Wanette Vargas, Jesse McClure, Katie Hemingway, Kyna Long, and Danielle Rioux for their assistance with this study, Dr. Brian Whitcomb for expert statistical advice, and Lynn Bengston and Jay Alexander for help with the figures. We are grateful to Dr. Wylie Vale for generously providing us with the CRF antisera.
Abbreviations
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- CRF
corticotropin-releasing factor
- -ir
-immunoreactive
- DAB
3,3′-diaminobenzidine
- FR1
fixed-ratio 1
- HPA
hypothalamic pituitary adrenal axis
- PD
postnatal day
References
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun G, Schwandt ML, Lindell SG, Kaschow JW, Suomi SJ, Goldman K, Higley JD, Heilig M. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci U.S.A. 2009;106:14593–14598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Badrinath S, Bush K, Boyd-Wickizer J, Anawalt B. Medical risks for women who drink alcohol. J Gen Intern Med. 1998;13:675–639. doi: 10.1046/j.1525-1497.1998.cr187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty ML, Wixey JA, Kesby J, Reinebrant HE, Colditz PB, Gobe B, Buller KM. Long-term losses of amygdala corticotropin-releasing factor neurons are associated with behavioural outcomes following neonatal hypoxia-ischemia. Behav Brain Res. 2010;208:609–618. doi: 10.1016/j.bbr.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Chou SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. Br J Addict. 1992;87:1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Orosan PG, Grant KE. Adolescent stress and coping: implications for psychopathology during adolescence. J Adolesc. 1993;16:331–349. doi: 10.1006/jado.1993.1028. [DOI] [PubMed] [Google Scholar]
- Courtney KF, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–50. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J Comp Neurol. 2003;463:434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of anxonal projections from the anterolateral area of the bed nucleus of the stria terminals. J Comp Neurol. 2004;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Donovan JE, Molina BS. Children’s introduction to alcohol use: sips and tastes. Alcohol Clin Exp Res. 2008;32:108–119. doi: 10.1111/j.1530-0277.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawal, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert D, Cippitelli A, Thorsell A, Le A, Hipskind P, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2, 6-dimethylimidazo [1, 2-b] pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in the adulthood in male rats. PlosOne. 2012;7(2):e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M. Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev. 2012;26:873–888. doi: 10.1016/j.neubiorev.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U.S.A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezova D, Jurankova E, Mosnarova A, Kriska M, Skultetyova I. Neuroendocrine response during stress with relation to gender differences. Acta Neurobiol Exp (Wars) 1996;56:779–785. doi: 10.55782/ane-1996-1183. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Backman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: overview of key findings, 2006. Bethesda, MD: 2007. [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JW, Binks SL, Li Y, Selvage D. The role of oestradiol in sexually dimorphic hypothalamic-pituitary-adrena axis responses to intracerebroventricular ethanol administration in the rat. J Neuroendocrinol. 2009;22:24–32. doi: 10.1111/j.1365-2826.2009.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress- induced relapse to alcohol-seeking behavior in rats. Psychopharm (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Le Mevel JC, Abitbol S, Beraud G, Maniey J. Temporal changes in plasma adrenocorticotropin concentration after repeated neurotropic stress in male and female rats. Endocrinology. 1979;105:812–817. doi: 10.1210/endo-105-3-812. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim CK, Rivier C. Nitric oxide stimulates ACTH secretion and the transcription of the genes encoding for NGFI-B, corticotropin-releasing factor, corticotropin-releasing factor receptor type 1, and vasopressin in the hypothalamus of the intact rat. J Neurosci. 1999;19:7640–7647. doi: 10.1523/JNEUROSCI.19-17-07640.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Kang SS, Lee S, Rivier C. Effects of ethanol on the regulation of corticotropin-releasing factor (CRF) gene expression. Mol Cell Neurosci. 2005;29:345–354. doi: 10.1016/j.mcn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharm. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharm (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Merlo-Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism NIAAA council approves definition of binge drinking. NIAAA Newsletter. 2004;(3):3. [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Rao YS, Pak TR. Binge-pattern alcohol exposure during puberty induces sexually dimorphic changes in genes regulating the HPA axis. Am J Physiol Endocrinol Methab. 2009;298:E320–E328. doi: 10.1152/ajpendo.00615.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. ISBN 3-900051-07-0, URL http://www.R-project.org/ [Google Scholar]
- Reinherz HZ, Giaconia RM, Lefkowitz ES, Pakiz B, Frost AK. Prevalence of psychiatric disorders in community population of older adolescents. J Am Ac Child Adol Psychiatry. 1993;32:369–377. doi: 10.1097/00004583-199303000-00019. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008a;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda K, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008b;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, Esser G, Rietschel M, Banaschewski T, Schumann G, Laucht M. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol. 2010;13:703–714. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Watts A. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Frontiers in Neuroendocrinology. 2005;26:109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Interactions of stress and CRF in ethanol-withdrawal induced anxiety in adolescent and adult rats. Alc Clin Exp Res. 2010;34:1603–1612. doi: 10.1111/j.1530-0277.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, Sterling ML, Council on Scientific Affairs, American Medical Association The neurocognitive effects of alcohol on adolescents and college students. Prev Med. 2005;40:23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharam (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]