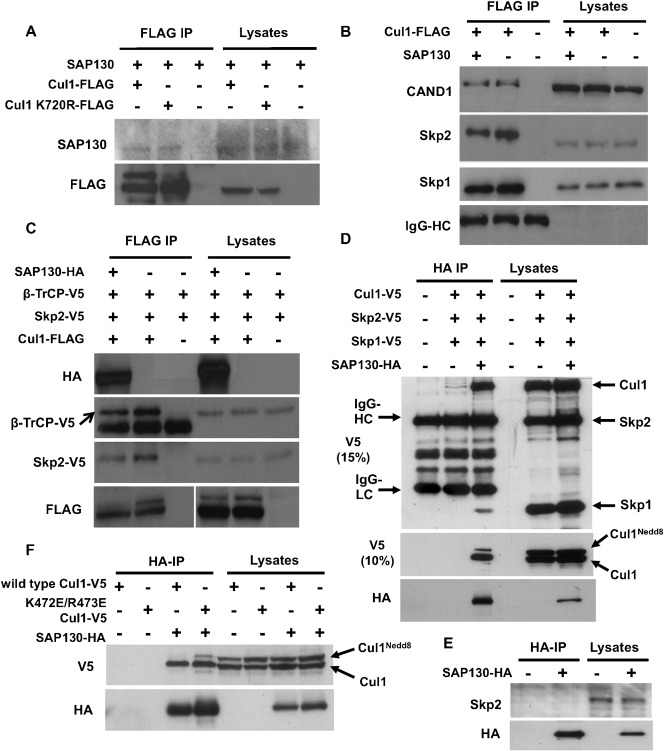
Fig. 1. SAP130 competes with Skp1-F-box protein for Cul1 binding.
(A) HEK293T cells were co-transfected with SAP130 and either Cul1-FLAG wild-type or Cul1 K720R-FLAG mutant. Lysates were FLAG immunoprecipitated and analyzed for SAP130 binding. (B) HEK293T cells were co-transfected with Cul1-FLAG and either SAP130 or empty vector. Lysates were FLAG immunoprecipitated and blotted with the indicated antibodies. (C) HEK293T cells were transfected as indicated with Skp2-V5, β-TrCP-V5, Cul1-FLAG and SAP130-HA. Following FLAG immunoprecipitation, binding of Skp2-V5 and β-TrCP to Cul1 was checked. (D) HEK293T cells were co-transfected with V5-tagged plasmids of Skp1, Skp2 and Cul1, and either SAP130-HA or empty vector; HA-immunoprecipitation was carried out. The immunoprecipitates and lysates were run on a 15% SDS gel to visualize Cul1-V5, Skp2-V5 and Skp1-V5 on the same Western blot by V5 immunoblotting. To visualize both the neddylated and unneddylated forms of Cul1-V5, the samples were re-run on a 10% SDS gel (middle panel). (E) HEK293T cells were transfected with SAP130-HA or empty vector, followed by immunoprecipitation of cell lysates using HA-agarose beads. Binding of endogenous Skp2 to SAP130-HA was detected by Western blotting of immunoprecipitates and lysates with Skp2 antibody. (F) HEK293T cells were transfected with the indicated expression plasmids. Cell lysates were subjected to HA immunoprecipitation and were blotted with V5 and HA antibodies.