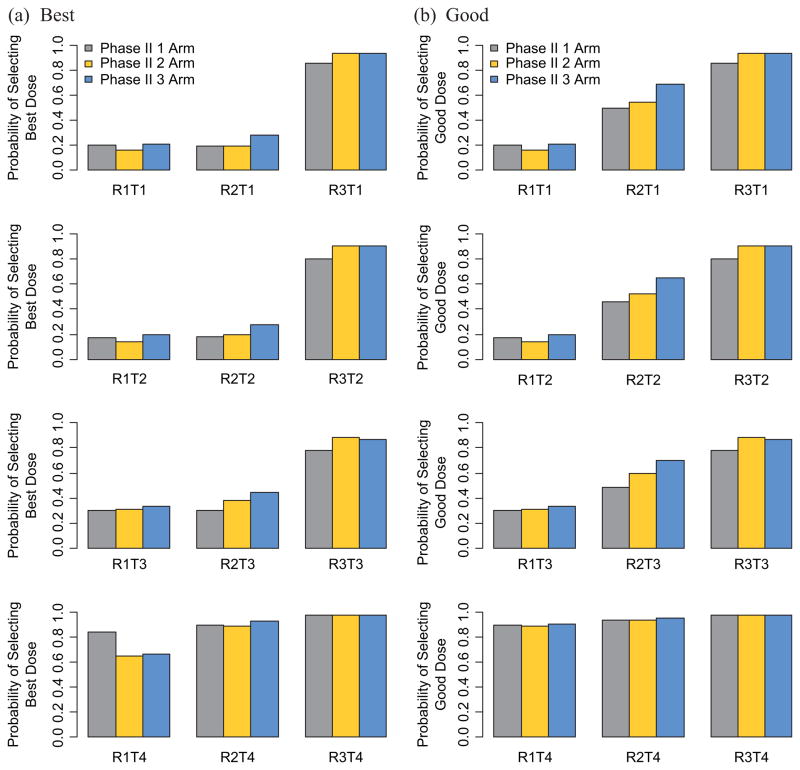
Figure 2.
Probability of stopping due to toxicity for the various scenarios and the three different phase II designs. For the single-arm phase II design, all patients are treated at the RD level; in the 2-arm phase II design, patients are randomized to RD and RD−; in the 3-arm phase II design, patients are randomized to RD, RD+, and RD−: (a) good dose level and (b) best dose level.
RD: phase II RD level; RD+ : dose level immediately above phase II RD level; RD−: dose level immediately below phase II RD level.