Abstract
β2-glycoprotein I (β2GPI)-dependent anticardiolipin autoantibodies (aCl) are associated with thrombosis and fetal loss. Some microbial pathogens can induce pathogenic antibodies cross-reactive with β2GPI. Sera from a significant percentage of periodontitis patients contain aCl, and some periodontal pathogens contain antigens with peptide sequences having homology to β2GPI. We hypothesized that antibodies raised against P. gingivalis (aPg) contain pathogenic aCl that induce fetal resorption. We immunized mice with β2GPI, P. gingivalis W83, or an arg-gingipain-defective mutant of P. gingivalis (HF18). IgG fractions of aPg were immunoabsorbed to remove aCl-like antibodies (abs-aPg). IgG fractions were administered intravenously into tail veins of mated BALB/c females at day 0 of pregnancy. At day 15, the proportions of fetal resorptions were evaluated. The prevalence of fetal loss was significantly greater in the aPg group than in the control IgG group (21.2% vs. 5.3%, p = .001), and greater in the aPg group than in the abs-aPg group (21.2% vs. 12%, p < .05). There were no fetal resorptions observed in the aPgHF18 group (p = .0005 compared with aPg, p = .17 compared with control). aPg antibody contains activity consistent with pathogenic aCl, and the antigen inducing the antibodies that cause increased fetal loss may be on the arg-gingipain protease of P. gingivalis.
Keywords: periodontal diseases, antibodies, pregnancy, fetus, molecular mimicry, mice
Introduction
Associations between periodontal disease and adverse pregnancy outcomes, including prematurity, low birthweight, preeclampsia, and fetal death, have been demonstrated in clinical studies and case reports (Vergnes and Sixou, 2007; Matevosyan, 2011). Although the biological processes that link these entities are not definitively known, it has been hypothesized that periodontal infections may directly or indirectly influence the fetal-placental unit (Offenbacher et al., 2006; Lin et al., 2007; Yokoyama et al., 2008; Han et al., 2009; Jared et al., 2009). It has been demonstrated that periodontal infections can affect systemic inflammation through transient bacteremias of oral origin and mobilization of inflammatory pathways such as C-reactive protein production by the liver, thus influencing systemic conditions such as atherosclerosis (Teles and Wang, 2011). It has therefore been hypothesized that such processes may affect the fetus by infection with oral organisms and promotion of inflammatory processes detrimental to fetal development and full-term pregnancies.
We have observed that patients with chronic and aggressive periodontitis demonstrate elevated levels of β2-glycoprotein I (β2GPI)-dependent anticardiolipin antibodies (aCl) compared with periodontally healthy individuals (Schenkein et al., 2003). These autoreactive antibodies are more typically found in patients with systemic lupus erythematosis (SLE) or the antiphospholipid syndrome (APS) and are thought to be at least in part responsible for adverse pregnancy outcomes such as fetal loss and fetal growth restriction in women with these diseases (Tripodi et al., 2011). However, periodontitis patients do not demonstrate pathology of the typical severity associated with autoimmune disease, though there are certainly associations of periodontal diseases with conditions of high prevalence in APS such as stroke, myocardial infarction, and prematurity.
The source of aCl in periodontitis is not well-understood, but it is known that some microbial pathogens can induce cross-reactive aCl antibodies that are pathogenic in animal models. It has also been demonstrated that Haemophilus influenzae, Neisseria gonorrhoeae, and tetanus toxoid can induce pathogenic cross-reactive aCl because they contain antigens with peptide sequences with sufficient similarity to a key sequence in β2GPI so as to induce aCl that demonstrate in vivo pathogenicity (Blank et al., 2002). We and others have identified such sequences in some oral bacteria and hypothesized that pathogens found in periodontal lesions that induce significant systemic antibody responses could induce pathogenic aCl (Schenkein et al., 2003; Wang et al., 2008; Chen et al., 2009).
In this study, we prepared antibodies to P. gingivalis, a key pathogen in both chronic and aggressive periodontitis that induces significant systemic antibody responses, and examined the aCl content and the ability of these antibodies to induce adverse outcomes in a mouse model of fetal growth.
Materials & Methods
This study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Antibody Production
Bacterial strains P. gingivalis W83 and P. gingivalis HF18 were grown in brain-heart infusion broth supplemented with hemin and menadione. P. gingivalis HF18 is an arg-gingipain-defective mutant of P. gingivalis W83 (Fletcher et al., 1995). Bacteria were washed in PBS, re-suspended in PBS containing alum at approximately 2 × 108/mL, and heated to 80oC for 10 min. A 50-μg quantity of bacterial suspension was injected intraperitoneally into groups of 10-20 naïve BALB/c mice, followed by 2 booster immunizations at weekly intervals. An additional group of mice was similarly immunized with purified β2GPI (Haematologic Technologies Inc., Cat# B2G1-0001, Essex Junction, VT, USA), or with alum suspended in PBS as a control. Mice were bled every 2 wks for 8 wks thereafter.
aCl Immunoabsorption and Purification of IgG from Mouse Sera
For generation of an aCl immunoabsorbant, Cardiolipin (Sigma-Aldrich, cat# C1649, St. Louis, MO, USA) was bound to Octyl-Sepharose (GE Healthcare, Cat# 17-0946-10, Piscataway, NJ, USA) according to the method of Pengo and Biasiolo (1993), followed by further incubation with purified β2GPI (100 µg/mL) for subsequent absorption of IgG preparations. The aCl immunoabsorbant was placed in either microfuge tubes, for large volumes, or in wells of MultiScreen-HTS Filter Plates. Mouse IgG samples were added to the aCl immunoabsorbant and then incubated with shaking for 60 min at room temperature. Filter plates were mounted onto sterile tissue culture plates for centrifugation at 500 × g for 10 min. The resulting supernatants were assayed for aCl and IgG.
IgG purification from mouse sera was accomplished with Protein G Sepharose (GE Healthcare BioSciences AB). Samples were adjusted to 1 M NaCl by the addition of NaCl, and pH was adjusted to 7.8 by the addition of 0.5 M Na2HPO4. A 5-mL quantity of each sample was applied to a 1-mL column of Protein G, equilibrated in 1 M NaCl, 0.05 M PO4, pH 7.8, and the column was washed until A280 was less than 0.02. Elution was accomplished by the addition of 0.1 M glycine, 0.2 M NaCl, pH 2.00. Eluted samples were immediately neutralized by the addition of 1 M NaOH. Subsequently, samples were dialyzed against PBS in dialysis cassettes (Thermo Scientific, Waltham, MA, USA). Fractions containing aCl were pooled and concentrated, where necessary, with Amicon Ultra centrifugal filters (Millipore, Billerica, MA, USA), then assayed for aCl. Protein concentration in IgG preparations was determined by absorbance at 280 nm.
Samples and column fractions were assayed for aCl with the Varelisa Cardiolipin IgG kit (Phadia, Portage, MI, USA), utilizing peroxidase-conjugated F(ab′)2 fragments of goat anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., Cat# 11-036-045, West Grove, PA, USA). Relative values for aCl in mouse IgG preparations were determined by linear regression analysis of dilutions of mouse IgG and expressed as the inverse of the serum dilution producing absorbance of 1.0 at 450 nm.
Assessment of Fetal Resorption
To determine the effects of antibody preparations on fetal loss, we intravenously infused purified IgG preparations (40 μg) into the tail veins of naïve mated BALB/c females at day 0 of pregnancy, and determined the percentage of fetal resorptions in the mouse groups as described previously (Blank et al., 2002). Fetuses were harvested at day 15 of pregnancy, and the proportion of resorbed fetuses (non-viable fetuses with weight < 0.1 g) was calculated. Experiments on groups of mice were repeated twice, and the data combined for analysis.
Statistical Analyses
Comparisons of the proportions of fetal resorptions between groups were determined by Fisher’s Exact Test. Differences in antibody titers were determined by ANOVA, with differences between paired groups assessed by Tukey’s post hoc test.
Results
Mice were immunized with β2GPI, P. gingivalis W83, P. gingivalis HF18, or alum. Portions of the antisera to β2GPI and P. gingivalis W83 were subsequently reacted with an immunoabsorbant consisting of cardiolipin complexed with β2GPI to remove antibodies cross-reactive with β2GPI, were recovered following centrifugation, and were analyzed for aCl immunoreactivity. Antisera raised against β2GPI contained significant aCl immunoreactivity while absorption with cardiolipin/β2GPI partially removed aCl immunoreactivity, demonstrating the effectiveness of the absorption technique (Table 1). Anti-P. gingivalis W83 also contained significant aCl immunoreactivity, which was similarly decreased following absorption with cardiolipin/ β2GPI, verifying that P. gingivalis induces immunoreactive aCl. However, antisera raised against the arg-gingipain-defective mutant of P. gingivalis W83 (strain HF18) contained only background levels of aCl equivalent to the control sera, suggesting that the P. gingivalis epitope inducing aCl activity could reside on the arg-gingipain protease.
Table 1.
aCl Concentrations in Antisera
| Antibody | Mean titer ± SD (N = 6) | Adjusted Pairwise p valuesa |
|---|---|---|
| Control | 290.4 ± 183.1 | |
| aβ2GPI | 2,437.8 ± 514.1 | p < .0001 vs. control |
| p < .0007 vs. β2GPI (absorbed) | ||
| aβ2GPI (absorbed) | 1,517.0 ± 253.1 | NSb vs. P. gingivalis (absorbed) |
| aP. gingivalis | 2,228.4 ± 283.7 | p < .0001 vs. control |
| p < .0007 vs. P. gingivalis (absorbed) | ||
| aP. gingivalis (absorbed) | 1,485.2 ± 279.2 | NS vs. β2GPI (absorbed) |
| aP. gingivalis HF18 | 227.8 ± 68.3 | NS vs. control |
Pairwise p values determined by ANOVA followed by Tukey’s post hoc test.
NS: not significant.
Mated female mice demonstrating vaginal plugs were passively administered 40 μg IgG of the antibody, absorbed antibody, and control IgG preparations at day 0 of pregnancy. At day 15, fetuses were harvested and weighed, and resorbed fetuses were noted. There were significantly more resorbed fetuses from mice administered aβ2GPI than fetuses from control mice (18.5% vs. 5.3%, p = .01) (Table 2), consistent with known pathological functions of these antibodies. Similarly, following administration of aPg W83 IgG to pregnant mice, there were significantly more resorbed fetuses noted at day 15 of gestation in comparison with those administered control IgG (21.3% vs. 5.3%, p = .001).
Table 2.
Fetal Loss Induced by aPg, aβ2GPI, and Absorbed aPg
| Group | Na | N Resorbedb | % Resorbed | p value (Fisher’s exact test) |
|---|---|---|---|---|
| Control | 75 | 4 | 5.33 | |
| aPgc | 127 | 27 | 21.26 | p = .001 vs. control |
| aβ2GPId | 65 | 12 | 18.46 | p = .01 vs. control |
| aPg-absd | 106 | 13 | 12.26 | p < .05 vs. aPg |
N: number of fetuses observed.
N resorbed: number of resorbed fetuses.
aPg: mouse IgG anti-P. gingivalis W83.
aβ2GPI: mouse IgG anti-β2-glycoprotein I.
Control mice were administered IgG from sham-immunized (alum alone) mice.
We next assessed the role of the aCl content of aPg in the induction of fetal resorption. aPg was absorbed to decrease the concentration of cross-reactive aCl antibody in the IgG preparations, and we compared the behavior of these antisera in the pregnancy model (Table 2). Immunoabsorption resulted in a significant decrease in fetal loss by aPg compared with absorbed aPg (21.3% vs. 12.2%, p < .05). The distribution of fetal resorptions among the pregnant mice administered aPg, absorbed aPg, or control IgG is shown in the Fig.
Figure.
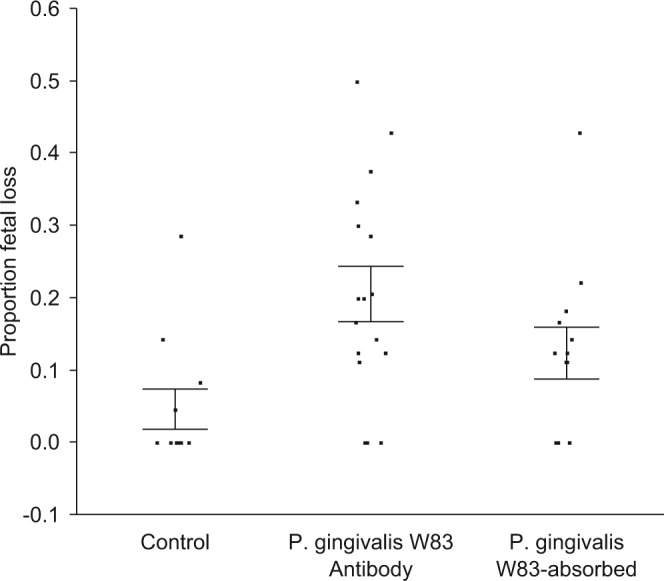
Proportion of resorbed fetuses noted for each pregnant mouse. Mated females observed to have vaginal plugs at day 0 were passively administered either IgG from anti-P. gingivalis W83, IgG from anti-P. gingivalis W83 absorbed with cardiolipin/β2GPI, or IgG from sham-immunized mice. At day 15 of gestation, fetuses were harvested, and the percentage of fully resorbed fetuses was calculated. Each point represents an individual mouse. Means ± standard error are shown.
Next, since an epitope capable of inducing antibodies that can cross-react with β2GPI has been identified on the arg-gingipain protease of P. gingivalis, we used IgG from antibody raised against an arg-gingipain-defective mutant of Pg W83, designated Pg HF18 (Table 3). These antibodies had no greater aCl titer than control sera (Table 1), and failed to induce fetal resorption (p = .0005 compared with PgW83; p = .17 compared with control; Fisher’s Exact Test).
Table 3.
Lack of Fetal Loss Induced by P. gingivalis HF18
p < .0001, Fisher’s Exact Test.
N: number of fetuses observed.
N resorbed: number of resorbed fetuses.
aPg: mouse IgG anti-P. gingivalis W83.
aHF18: mouse IgG antibody against P. gingivalis HF18 (arg-gingipain defective mutant strain).
Control mice were administered IgG from sham-immunized (alum alone) mice.
Discussion
It has been observed that there are associations of preterm birth with exposure to and levels of periodontal pathogens. P. gingivalis has been localized to placentas of patients with preterm delivery and chorioamnionitis (Katz et al., 2009) and within chorionic tissues (Hasegawa-Nakamura et al., 2011), and may be present in higher postpartum concentrations in the oral cavity following preterm delivery (Lin et al., 2007).
Antibody against P. gingivalis, though prevalent in patients with periodontitis and frequently present at high titer, does not appear to be associated with preterm birth. Levels of aPg have in fact been observed in some studies to be inversely related to risk for prematurity (Lin et al., 2007; Ebersole et al., 2009). Such studies support a hypothesis that low antibody levels favor increased exposure of the fetal tissues and fetus to periodontal pathogens.
Analysis of the data in this paper suggests the possibility that a specific subset of antibodies to P. gingivalis that is present as a small proportion of the overall aPg content may be partly responsible for some of the associations noted between periodontal diseases and adverse pregnancy outcomes. In contrast to an assessment of antibody titer against the whole bacteria, the data analysis illustrates that cross-reactive antibody constituting a very low proportion of the total antibody reactive with P. gingivalis may have a profound biological effect in this pregnancy model. It has been demonstrated that other micro-organisms can in fact induce the production of pathogenic antiphospholipid antibodies such as aCl. Blank and co-workers showed that mice immunized with Haemophilus influenzae, Neisseria gonorrhoeae, or tetanus toxoid induced high titers of aβ2GPI molecules with specificity for the TLRVYK peptide sequence recognized by pathogenic aCl (Blank et al., 2002). These antibodies induced APS-like symptoms when passively infused into pregnant mice. In human disease, associations between bacterial and viral infections and APS have been noted, but only in a few cases are cross-reactive aCl shown to be reactive with β2GPI and produce symptoms consistent with APS. Thus, in most cases, the etiological significance of infection in APS or in non-autoimmune diseases with symptoms of rheumatic diseases remains controversial (Artenjak et al., 2012).
Analysis of our previous data demonstrated that the proportion of individuals with chronic or aggressive periodontitis testing positive for aCl is greater than in periodontally healthy individuals (Schenkein et al., 2003), suggesting an oral source of immunogen in these patients, since none had a history of autoimmune disease or other infection. The current study shows that, indeed, the periodontal pathogen P. gingivalis may induce these antibodies. We believe that the antigen capable of inducing aCl in P. gingivalis is present in its arg-gingipain protease. The peptide sequence TLRIYT of arg-gingipain is identified as substantially homologous to TLRVYK in the Swiss-prot database, and antibodies raised against TLRIYT cross-react with antibodies raised against TLRVYK of β2GPI (Chen et al., 2009). We compared fetal resorption between antibodies raised to P. gingivalis and an arg-gingipain-defective mutant strain HF18, and found that the antibody against the arg-gingipain-defective mutant failed to induce fetal loss.
Wang and co-workers previously demonstrated that Aggregatibacter actinomycetemcomitans may induce aCl-like antibodies, due to the presence of the hexapeptide SIRVYK in leukotoxin c that could induce antibodies cross-reactive with the TLRVYK peptide of β2GPI (Wang et al., 2008). Indeed, they observed elevated anti-SIRVYK in sera from patients with chronic and aggressive periodontitis. Further, they observed correlations between anti-CL and potentially cross-reactive peptides found in other periodontal pathogens, including TLRIYT of P. gingivalis arg-gingipain and TLALYK in the phosphoglycerate kinase of Treponema denticola in patients with Buerger’s disease (Chen et al., 2009). Thus, at least 3 periodontal pathogens may have the capability of inducing mutually cross-reactive antibodies with β2GPI, accounting for the presence of elevated levels of aCl in sera from individuals with periodontitis.
Molecular mimicry by periodontal bacteria, and especially by P. gingivalis, certainly has precedent. P. gingivalis, via its GroEl antigen, can induce antibodies reactive with heat-shock proteins (Hinode et al., 1998; Choi et al., 2004; Yamazaki et al., 2004), and this is a proposed mechanism linking periodontitis with systemic disease. It is hypothesized that anti-GroEl reacts with HSP-60 on endothelial cells, enhancing vascular inflammatory responses in atherosclerosis and other inflammatory conditions. Interestingly, aCl may have similar biological effects related to atherosclerosis (Narshi et al., 2011), since autoimmune aCl promotes endothelial inflammatory responses and opsonizes low-density lipoproteins, and thus may accelerate the development of atherosclerosis in patients with SLE and APS. Our recent studies in fact demonstrate that human aCl from patients with periodontitis promote cytokine release from human vascular endothelial cells (Schenkein et al., 2013).
In summary, it appears that P. gingivalis is capable of inducing antibodies cross-reactive with β2GPI, the target antigen of autoimmune aCl, and that these antibodies promote fetal resorption in a mouse pregnancy model. This suggests a hypothesis that molecular mimicry by this mechanism may influence systemic conditions that are epidemiologically related to periodontal infections.
Acknowledgments
The authors acknowledge Mr. Rennie Berry for his excellent technical assistance and Dr. Al M. Best for assistance with the statistical analyses.
Footnotes
This work was supported by the National Institute of Dental and Craniofacial Research (grant RO1DE018125) and the National Institute on Minority Healthy and Health Disparities (grant P60MD002256).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Artenjak A, Lakota K, Frank M, Cucnik S, Rozman B, Bozic B, et al. (2012). Antiphospholipid antibodies as non-traditional risk factors in atherosclerosis based cardiovascular diseases without overt autoimmunity. A critical updated review. Autoimmun Rev 11:873-882. [DOI] [PubMed] [Google Scholar]
- Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, et al. (2002). Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J Clin Invest 109:797-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Nagasawa T, Wara-Aswapati N, Ushida Y, Wang D, Takeuchi Y, et al. (2009). Association between periodontitis and anti-cardiolipin antibodies in Buerger disease. J Clin Periodontol 36:830-835. [DOI] [PubMed] [Google Scholar]
- Choi JI, Chung SW, Kang HS, Rhim BY, Park YM, Kim US, et al. (2004). Epitope mapping of Porphyromonas gingivalis heat-shock protein and human heat-shock protein in human atherosclerosis. J Dent Res 83:936-940. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Novak MJ, Michalowicz BS, Hodges JS, Steffen MJ, Ferguson JE, et al. (2009). Systemic immune responses in pregnancy and periodontitis: relationship to pregnancy outcomes in the Obstetrics and Periodontal Therapy (OPT) study. J Periodontol 80:953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher HM, Schenkein HA, Morgan RM, Bailey KA, Berry CR, Macrina FL. (1995). Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect Immun 63:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. (2009). Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol 47:38-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa-Nakamura K, Tateishi F, Nakamura T, Nakajima Y, Kawamata K, Douchi T, et al. (2011). The possible mechanism of preterm birth associated with periodontopathic Porphyromonas gingivalis. J Periodontal Res 46:497-504. [DOI] [PubMed] [Google Scholar]
- Hinode D, Nakamura R, Grenier D, Mayrand D. (1998). Cross-reactivity of specific antibodies directed to heat shock proteins from periodontopathogenic bacteria and of human origin [corrected]. Oral Microbiol Immunol 13:55-58. [DOI] [PubMed] [Google Scholar]
- Jared H, Boggess KA, Moss K, Bose C, Auten R, Beck J, et al. (2009). Fetal exposure to oral pathogens and subsequent risk for neonatal intensive care admission. J Periodontol 80:878-883. [DOI] [PubMed] [Google Scholar]
- Katz J, Chegini N, Shiverick KT, Lamont RJ. (2009). Localization of P. gingivalis in preterm delivery placenta. J Dent Res 88:575-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Moss K, Beck JD, Hefti A, Offenbacher S. (2007). Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J Periodontol 78:833-841. [DOI] [PubMed] [Google Scholar]
- Matevosyan NR. (2011). Periodontal disease and perinatal outcomes. Arch Gynecol Obstet 283:675-686. [DOI] [PubMed] [Google Scholar]
- Narshi CB, Giles IP, Rahman A. (2011). The endothelium: an interface between autoimmunity and atherosclerosis in systemic lupus erythematosus? Lupus 20:5-13. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG, et al. (2006). Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol 107:29-36. [DOI] [PubMed] [Google Scholar]
- Pengo V, Biasiolo A. (1993). Purification of anticardiolipin and lupus anticoagulant activities by using cardiolipin immobilized on agarose beads. Thromb Res 72:423-430. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Berry CR, Burmeister JA, Brooks CN, Barbour SE, Best AM, et al. (2003). Anti-cardiolipin antibodies in sera from patients with periodontitis. J Dent Res 82:919-922. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Sabatini R, Koertge TE, Brooks CN, Purkall DB. (2013). Anti-cardiolipin from periodontitis patients induces MCP-1 production by human umbilical vein endothelial cells. J Clin Periodontol 40:212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles R, Wang CY. (2011). Mechanisms involved in the association between periodontal diseases and cardiovascular disease. Oral Dis 17:450-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi A, de Groot PG, Pengo V. (2011). Antiphospholipid syndrome: laboratory detection, mechanisms of action and treatment. J Intern Med 270:110-122. [DOI] [PubMed] [Google Scholar]
- Vergnes JN, Sixou M. (2007). Preterm low birth weight and maternal periodontal status: a meta-analysis. Am J Obstet Gynecol 196:135.e1-7. [DOI] [PubMed] [Google Scholar]
- Wang D, Nagasawa T, Chen Y, Ushida Y, Kobayashi H, Takeuchi Y, et al. (2008). Molecular mimicry of Aggregatibacter actinomycetemcomitans with beta2 glycoprotein I. Oral Microbiol Immunol 23:401-405. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Ohsawa Y, Itoh H, Ueki K, Tabeta K, Oda T, et al. (2004). T-cell clonality to Porphyromonas gingivalis and human heat shock protein 60s in patients with atherosclerosis and periodontitis. Oral Microbiol Immunol 19:160-167. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Hinode D, Yoshioka M, Fukui M, Tanabe S, Grenier D, et al. (2008). Relationship between Campylobacter rectus and periodontal status during pregnancy. Oral Microbiol Immunol 23:55-59. [DOI] [PubMed] [Google Scholar]


