Figure 9.
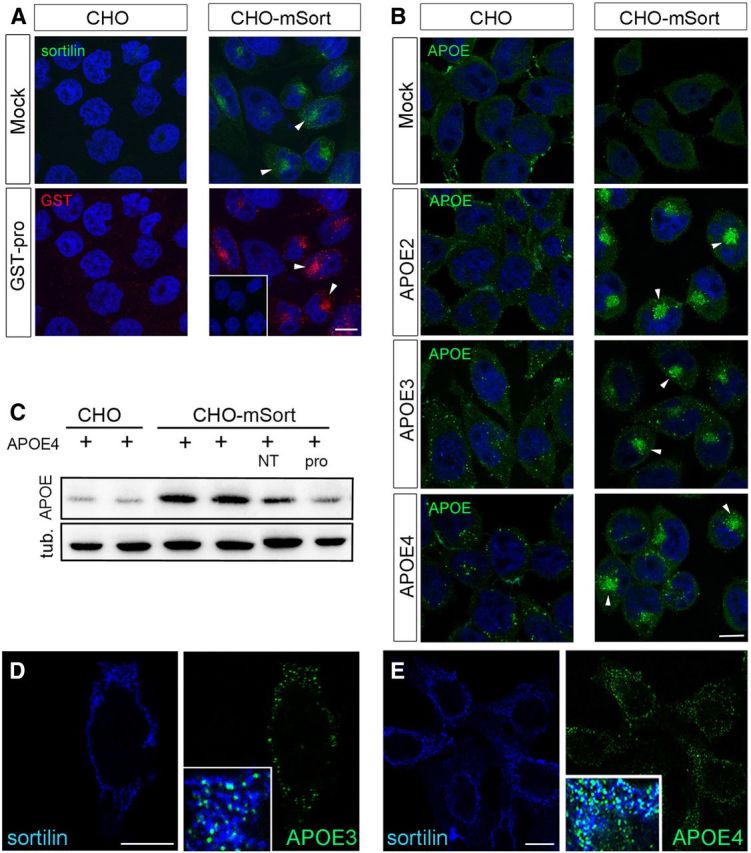
Sortilin mediates cellular uptake of human APOE. A, B, Replicate layers of parental CHO cells or CHO cells overexpressing murine sortilin (CHO–mSort) were incubated with blank medium (mock), with medium containing 500 nm of the pro-domain of NGF fused to GST (GST–pro), or with conditioned media from HEK293 cells transfected with expression constructs for human APOE2, APOE3, or APOE4. Receptor-dependent uptake of ligands was determined by immunodetection of sortilin (A), GST–pro (A), as well as APOE (B). Arrowheads highlight the localization of receptor and ligands in the perinuclear region of the cells. The inset in A indicates lack of ligand uptake in cells treated with GST instead of GST–pro. Scale bars, 4 μm. C, Detection of APOE and tubulin (loading control) in extracts of parental CHO or CHO–mSort cells incubated for 30 min with lipidated recombinant APOE4. When indicated, the medium also contained 10 μm NT or GST–Pro (pro). D, E, Detection of sortilin and APOE3 (D) and sortilin and APOE4 (E) on the surface of nonpermeabilized CHO–mSort cells by immunocytochemistry. Insets in D and E show the merged pictures, with white color indicating colocalization of sortilin (blue) and APOE (green) on the cell surface. Scale bars, 4 μm.
