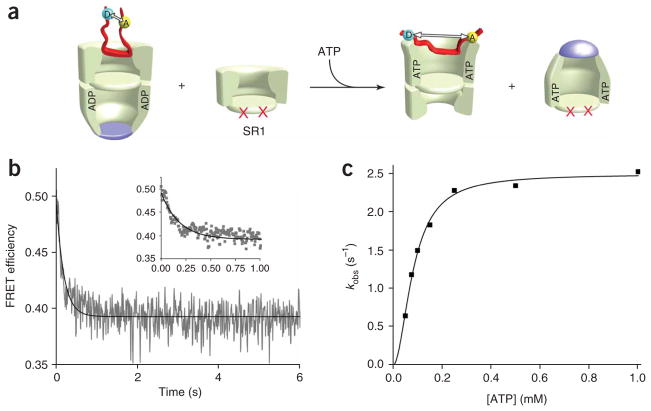
Figure 4.
ATP binding alone to a Rubisco-occupied trans ring drives a forced conformational expansion of the non-native protein. (a) Schematic of a FRET experiment designed to follow conformational changes in a non-native substrate protein upon ATP binding alone to the substrate-loaded trans ring of an ADP bullet. In this case, a large excess of a single-ringed GroEL variant SR1 (ref. 21) was added as a GroES trap. SR1 contains a set of equatorial mutations that prevent assembly of a double-ringed complex (indicated by red crosses). SR1 binds ATP and GroES, executes a single round of ATP hydrolysis and then stalls, failing to release GroES and ADP21. (b) The allosteric transition triggered by ATP alone results in the forced conformational expansion of the non-native protein. Stopped-flow FRET experiments were conducted in essentially the same manner as in Figure 3, except that SR1 was present in a 20-fold excess (1.2 μM) to prevent GroES binding to the substrate-loaded GroEL ring. (c) The observed rate of Rubisco expansion on the trans ring of an ADP bullet at different concentrations of ATP is shown. Each data point in the plot represents a single exponential fit to the observed change in FRET efficiency of the labeled Rubisco calculated from the average of n = 10 experimental replicates. The solid line shows a fit of the data to the Hill equation with kobs = 2.49 ± 0.06 s−1, K1/2 = 82.6 ± 3.7 μM and nH = 1.9 ± 0.2.