Figure 5.
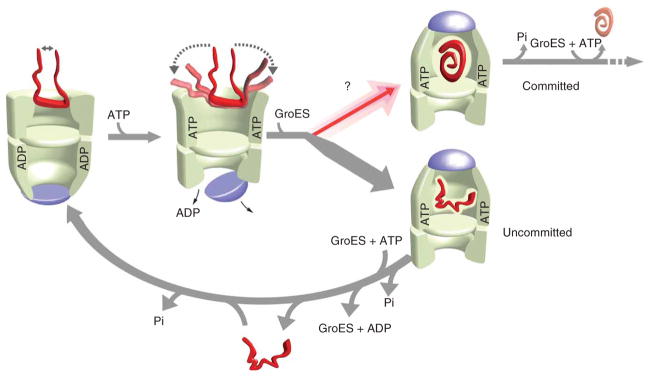
A functional model for substrate-protein unfolding by GroEL. Partial substrate unfolding by GroEL occurs in two phases: (i) passive unfolding upon protein capture by a GroEL ring; and (ii) forced unfolding upon ATP binding to a substrate-loaded trans ring. Although substrate confinement within the GroEL–GroES cavity enhances folding, the GroEL–GroES cavity exists for only a short period of time (~15–20 s at 25 °C) during a functional GroEL cycle17,19,20,25,27,34–36. Substrate unfolding provides, in principle, a method for driving a non-native substrate protein to a higher level of its folding free energy landscape, opening efficient folding paths (committed) that are not readily accessible to more compact states (uncommitted)14,15. Substrate-protein unfolding before encapsulation might substantially enrich the fraction of protein that commits to a productive folding path with each round of the GroEL cycle. ATP binding to the trans ring of the asymmetric complex, once hydrolysis of ATP within the GroEL–GroES cavity is complete (Pi), induces disassembly of the GroEL–ADP–GroES complex and ejection of the substrate protein, folded or not17,26,27,30. Uncommitted substrate must be recaptured on the trans ring of an ADP bullet for another round of unfolding and encapsulation (lower arrow). Two full rounds of ATP hydrolysis, one in each ring, followed by a round of substrate capture on the trans ring, returns the asymmetric ATP-containing complex to the starting ADP-bullet state.
