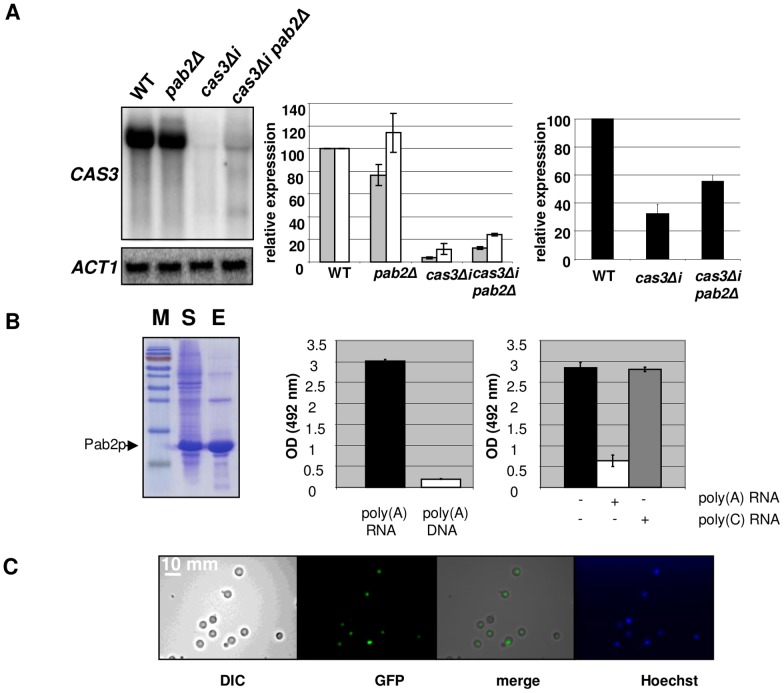
Figure 4. Pab2p is a nuclear poly(A) binding protein necessary for intron-dependent regulation of CAS3 expression.
A. Left panel. Northern blot experiment results showing the effect of PAB2 deletion on CAS3 mRNA accumulation. Central panel. Quantification of CAS3 mRNA accumulation in the different genetic backgrounds and normalized to ACT1 mRNA levels. These quantifications were done using RNA extracted from intact cells (grey bars) or from nuclei enriched fractions (white bars). The reported values are the means ± SD of three independent experiments. Right panel. Complementation of the capsule structure phenotypes as measured using the peroxidase-linked anti-capsule Mab 302. B. Left panel. Purification of a recombinant His-tagged-Pab2p in E. coli. 10% SDS/PAGE gel stained with Coomassie blue of E. coli lysate soluble supernatant (lane S), purified protein after affinity Column purification (lane E). Central panel. Pab2p binding assays. Affinity of a recombinant His-tagged Pab2p produced in E. coli to poly(A) was tested in a 96 well-plate format. Each well was coated with a poly(A) 30-mer oligonucleotide RNA. After incubation and washes, the quantity of bound proteins was estimated using an anti-His peroxidase linked monoclonal antibody. The affinity of Pab2p to poly(A) RNA and to poly(A) DNA were compared. Right panel. Competition assays in which 10 µM of unlabeled poly(A) or poly(C) (RNA) were added to the protein solution. C. Cells expressing the GFP-tagged version of the Pab2 protein were grown on glucose at 30°C and examined by epifluorescence or under bright field. Hoechst staining was used as control.