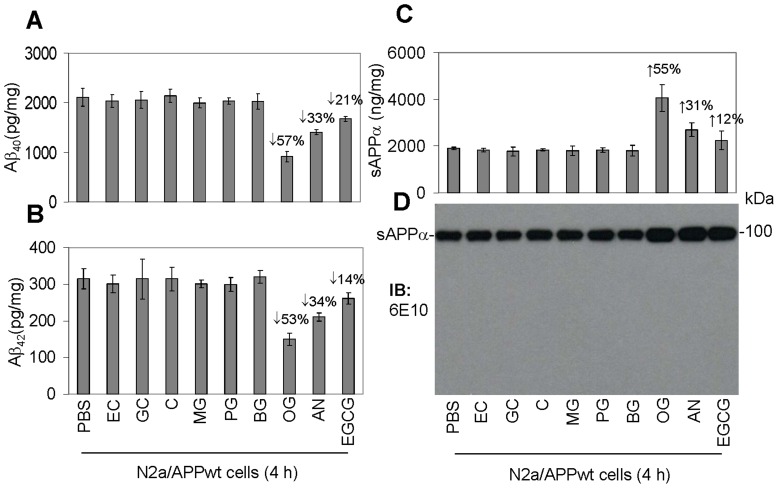
Figure 1. Characterization of gallate moiety in increasing amyloid precursor protein (APP) α-proteolysis and reducing amyloid-β (Aβ) generation.
Given (−)-epigallocatechin-3 gallate (EGCG) significantly reduces Aβ generation from N2a/APPsw cells, we screened other gallate containing phenolic compounds, namely (−)-gallocatechin (GC), methyl gallate (MG), propyl gallate (PG), butyl gallate (BG), octyl gallate (OG), and atranorin (AN) (an ester of gallic acid) and two non-gallate containing compounds, (−)-epicatechin (EC) and (−)-catechin (C), for their ability to reduce Aβ generation (Table 1). (A-D) N2a cells overexpressing wild-type APP (N2a/APPwt) were plated in 24 well-plates (200,000 cells/well) and treated with each of these compounds at 10 µM (our previous studies indicated that this is the minimum concentration of EGCG required to noticeably inhibit Aβ production in N2a/APPsw cells) in addition to PBS control. Data are represented as pg of Aβ40, Aβ42 (A, B) or ng of sAPPα (C) in the conditioned media secreted throughout the 4 h of administration for each compound, normalized to intracellular protein (mg). sAPPα ELISA results were further supported by immunoblotting analysis (IB) using an anti-Aβ1–17 antibody (6E10) (D). One-way ANOVA followed by post hoc comparison revealed significant differences when comparing EGCG, AN and OG to each of other compounds. These results are representative of three independent experiments with three replicates for each condition. We have summarized these data in Table 1. Together, they suggest that the gallate moiety may be an important functional component in EGCG, AN and OG, consequently playing a role in reducing Aβ generation and elevating APP α-processing. We have replicated these results in cells overexpressing the Swedish mutant form of APP (N2a/APPsw cells, data not shown).