Figure 1. Action of V. parahaemolyticus QS systems.
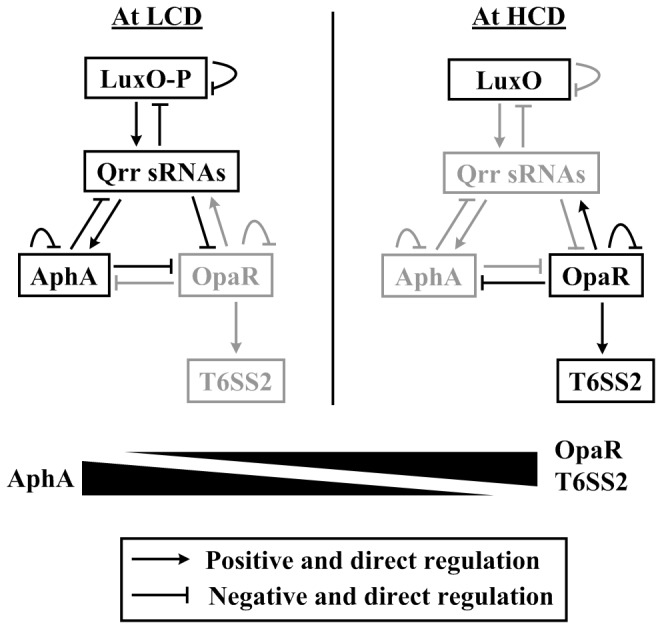
The regulatory associations between LuxO, Qrr sRNAs, AphA, and OpaR were summarized with the integration of relevant observations in V. parahaemolyticus [4,5] and closely related V. harveyi [3,32–40]. The grey fonts denoted the inhibited production of relevant proteins or the cease of relevant regulatory cascades. At LCD, low concentrations of autoinducers lead to phosphorylation of LuxO (LuxO-P), and LuxO-P activates expression of Qrr sRNA genes [32,33]. Redundant Qrr sRNAs promote AphA translation and, meanwhile, inhibit OpaR translation [34–36]. AphA further represses opaR transcription [3,5]. Overproduced AphA feeds back to inhibit transcription of qrr2-3 and its own gene [3,5]. In addition, over-production of Qrr sRNAs and LuxO-P triggers three additional feedback regulatory loops: i) LuxO-P represses transcription of its own gene, ii) Qrr sRNAs inhibits luxO translation, and iii) Qrr sRNAs repress translation of luxMN encoding the membrane-anchoring autoinducer-binding receptor protein LuxM and its cognate receptor LuxN [37–39]. The above feedbacks will contribute to control the LuxO-P, Qrr and AphA levels within the physiological states. At HCD, high concentrations of autoinducers reverse the phosphate flow in the circuit, leading to dephosphorylation of LuxO. Dephosphorylated LuxO is inactive as a regulator, leading to cessation of Qrr sRNA production; thus, there is no production of AphA but OpaR translation occurs [34,35]. OpaR in turns represses aphA transcription [4,40] but stimulates T6SS2 transcription (this study), and it also feeds back to inhibit its own expression [4,40]. OpaR is also able to activate the qrr2-4 transcription, leading to rapid down-regulation of opaR [4,40]; this OpaR-qrr feedback dramatically accelerates transition from HCD to LCD, but it has no effect on QS behaviors at steady-state LCD or HCD [41]. Taken together, there is the reciprocal gradients of cellular AphA and OpaR levels during transition between LCD and HCD, and AphA and OpaR act as the master QS regulators at LCD and HCD, respectively. In addition, T6SS2 transcription enhances in a gradient manner with transition from LCD to HCD, which is coordinately controlled by AphA and OpaR.
