Abstract
Limited axonal plasticity within the central nervous system (CNS) is a major restriction for functional recovery after CNS injury. The small GTPase RhoA is a key molecule of the converging downstream cascade that leads to the inhibition of axonal re-growth. The Rho-pathway integrates growth inhibitory signals derived from extracellular cues, such as chondroitin sulfate proteoglycans, Nogo-A, myelin-associated glycoprotein, oligodendrocyte-myelin glycoprotein, Ephrins and repulsive guidance molecule-A, into the damaged axon. Consequently, the activation of RhoA results in growth cone collapse and finally outgrowth failure. In turn, the inhibition of RhoA-activation blinds the injured axon to its growth inhibitory environment resulting in enhanced axonal sprouting and plasticity. This has been demonstrated in various CNS-injury models for direct RhoA-inhibition and for downstream/upstream blockade of the RhoA-associated pathway. In addition, RhoA-inhibition reduces apoptotic cell death and secondary damage and improves locomotor recovery after experimental spinal cord injury (SCI). Unexpectedly, a subset of “small molecules” from the group of non-steroid anti-inflammatory drugs, particularly the FDA-approved ibuprofen, has recently been identified as (1) inhibiting RhoA-activation, (2) enhancing axonal sprouting/regeneration, (3) protecting “tissue at risk” (neuroprotection) and (4) improving motor recovery confined to realistic therapeutical time-frames in clinically relevant SCI models. Here, we survey the effect of small-molecule-induced RhoA-inhibition on axonal plasticity and neurofunctional outcome in CNS injury paradigms. Furthermore, we discuss the body of preclinical evidence for a possible clinical translation with a focus on ibuprofen and illustrate putative risks and benefits for the treatment of acute SCI.
Keywords: Plasticity, Axonal damage, Spinal cord, Clinical trial, Ibuprofen
Introduction
The options for the restoration of neurological functions after central nervous system (CNS) injury are strictly limited. A major reason for the devastating prognosis of severe CNS injury with regard to functional recovery is the incapability of CNS axons to re-grow. “Molecular barriers” located in the axon environment have been identified as impediments for neuronal regeneration in the CNS. These molecules within the scar tissue and myelin are up-regulated after CNS injury and interact with various receptors on the surface of the injured axon. The signals from most of these receptors converge to RhoA, a key molecule in the growth inhibitory cascade. Interference with Rho-activation blocks the stop-signal transduction and finally promotes axonal plasticity/regeneration and thus functional recovery. Despite substantial experimental progress, no pharmacological treatment is available for the SCI patient as yet, except within early phase clinical trials. Excellent reviews exist, both on the extrinsic regeneration barriers in the CNS (e.g., Xie and Zheng 2008; Yiu and He 2006) and on the molecular rationale of Rho-inhibition on the promotion of functional recovery after CNS injury (e.g., McKerracher and Higuchi 2006; Mueller et al. 2005; Rossignol et al. 2007). In addition to their classical role as cyclooxygenase (COX) inhibitors, individual “small molecules” from the group of non-steroidal anti-inflammatory drugs (NSAIDs) have been identified to effectively block Rho-activation. This review updates the evidence for Rho-inhibition, with emphasis on the recently demonstrated NSAID-mediated effects, as a relevant therapeutic approach to enhance the recovery of neurological function after traumatic spinal cord injury (SCI).
Molecular players within the Rho-pathway
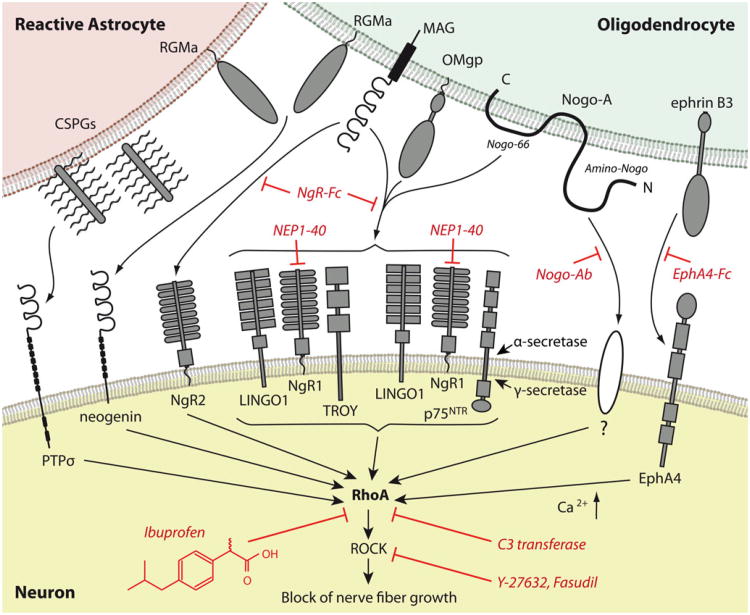
Chondroitin sulfate proteoglycans (CSPGs) synthesized by reactive astrocytes within the extracellular matrix (ECM) of the glial scar are potent inhibitors of axonal regrowth (for reviews, see Bartus et al. 2011; Morgenstern et al. 2002). Their signal is transduced into the axon through the receptor PTPσ (Shen et al. 2009). Main inhibitory myelin components include oligodendrocyte-myelin glycoprotein (OMgp), myelin-associated glycoprotein (MAG) and the Nogo-66 segment from the Nogo-A molecule signal through the Nogo-receptor complex that consists in the Nogo receptor NgR1, LINGO1 and p75NTR or TROY (Fournier et al. 2001; Kottis et al. 2002; Liu et al. 2002; Mi et al. 2004; Shao et al. 2005; Wang et al. 2002; for reviews, see Liu et al. 2006; McGee and Strittmatter 2003). The receptor for the Amino-Nogo segment is unknown (Fournier et al. 2001; Hu and Strittmatter 2008; for reviews, see Buchli and Schwab 2005; Schwab et al. 2006; Schweigreiter and Bandtlow 2006). The action of Amino-Nogo depends on specific integrin engagement, with alpha-V, alpha-5 and alpha-4 integrin-containing complexes being selectively sensitive to disruption by Amino-Nogo (Hu and Strittmatter 2008). Whereas these integrins are required for Amino-Nogo inhibition, their role does not involve the direct binding of Amino-Nogo to the integrins and unidentified intermediate Amino-Nogo-binding proteins must be involved. MAG also binds with higher affinity to the Nogo receptor homolog NgR2 (Robak et al. 2009). The MHCI-interacting protein, PirB, might serve as an additional binding site for Nogo-66, MAG and OMgp but its axon-regeneration role in vivo appears limited (Atwal et al. 2008; Fujita et al. 2011; Huebner et al. 2011; Nakamura et al. 2011; Omoto et al. 2010). Repulsive guidance molecules that exert growth inhibition in the mature CNS include EphrinB3 or RGMa, which bind to EphA4 (Benson et al. 2005) or neogenin (Conrad et al. 2007; Rajagopalan et al. 2004), respectively (Fig. 1). The converging intra-axonal inhibitory signals from these molecules activate RhoA. Subsequently, the Rho-associated coiled kinase (ROCK) is activated. ROCK has effects on the cytoskeleton of the nerve fiber growth cone and induces its collapse (for a review, see Schmandke et al. 2007). Inhibition of the Rho-cascade blocks the integration of the growth-inhibitory signal into the injured axon, resulting in propagated sprouting following axonal injury. This has been demonstrated for various molecular targets within the Rho-cascade. Nogo-A antibodies block Nogo-binding domains and thereby reduce RhoA-activation and promote axonal regrowth (Liebscher et al. 2005; Merkler et al. 2001; Muellner et al. 2008; Seymour et al. 2005). The NgR1 antagonist peptide NEP1-40 competes against Nogo-66 for binding to NgR1 (GrandPré et al. 2002; Li and Strittmatter 2003) and the soluble Nogo receptor fusion proteins NgR-Fc or NgROMNI-Fc block binding to NgR1 and NgR2 (Barton et al. 2003; Fournier et al. 2002; Lee et al. 2004; Li et al. 2004; Li and Strittmatter 2003; Robak et al. 2009; Wang et al. 2006). By analogy, Ephrin binding to EphA4 can be blocked by soluble unclustered EphA4-Fc (Goldshmit et al. 2011). The ROCK-inhibitors Y-27632, fasudil and others target the Rho downstream pathway. RhoA is selectively blocked by the exoenzyme C3 transferase derived from Clostridium botulinum (for reviews, see McKerracher and Higuchi 2006; Mueller et al. 2005). Individual “small molecules” from the group of NSAIDs have been identified to inhibit Rho-activation (Dill et al. 2010; Fu et al. 2007; Wang et al. 2009; Zhou et al. 2003) as detailed in this review (Fig. 1).
Fig. 1.
Inhibitory molecules and therapeutic targets within the Rhopathway. Diverse growth inhibitory molecules from myelin and reactive astrocytes interact with various receptors signaling through a converging downstream pathway within the axon after injury to the central nervous system. The activation of RhoA represents a key mechanism of this cascade. NgR1 forms a receptor complex with LINGO1 and p75NTR or TROY, which is required for Nogo-66 signaling. Cleavage of p75NTR through α-secretase and γ-secretase leads to the release of its intracellular domain, which transmits the inhibitory signal. The Amino-Nogo sequence of Nogo-A activates RhoA through an unknown receptor. The increase in intracellular calcium is involved in RhoA-activation. Interference with RhoA-activation blocks the integration of the inhibitory signal into the injured axon and results in axonal re-growth. At the upstream level, Nogo antibodies prevent the inhibition of Nogo-A-mediated outgrowth. Soluble NgR-Fc or NgROMNI -Fc block the binding of myelin inhibitors to NgR1 or NgR2 and the NgR1 agonist NEP1-40 also interferes with binding to NgR1. Ephrin signaling can be blocked by unclustered EphA4-Fc. RhoA-activation itself is inhibited by C3 transferase and by “small molecules”, such as the NSAID ibuprofen. Y27623 and fasudil inhibit the downstream target ROCK (CSPGs chondroitin sulfate proteoglycans, MAG myelin-associated glycoprotein, OMgp oligodendrocyte-myelin glycoprotein, ROCK Rho-kinase, RGMa repulsive guidance molecule–A)
Selective Rho-blockade
C3 transferase has been demonstrated in vitro to enhance axonal outgrowth in the presence of inhibitory matrix (Boato et al. 2010; Dergham et al. 2002; Fournier et al. 2003; Jin and Strittmatter 1997; Lehmann et al. 1999; Monnier et al. 2003) and to protect neurons from death (Julien et al. 2008) and from p75NTR-mediated amyloid β (Aβ) toxicity (Chacon et al. 2011). In addition, C3 transferase has been detected to antagonize tumor necrosis factor-α (TNF-α)-mediated apoptosis in oligodendrocytes (Xing et al. 2011). In vivo, local application of C3 transferase leads to axonal spouting after CNS injury. This was first demonstrated in an optic nerve crush model (Lehmann et al. 1999). Following experimental spinal cord transection (Boato et al. 2010; Dergham et al. 2002; Lord-Fontaine et al. 2008), contusion (Lord-Fontaine et al. 2008) and compression (Boato et al. 2010), C3 transferase application results in enhanced sprouting of corticospinal tract (CST) fibers (Boato et al. 2010; Dergham et al. 2002) and serotonergic neurites (Boato et al. 2010) and in improved locomotion (Boato et al. 2010; Lord-Fontaine et al. 2008; Schwab et al. 2002). In addition, a neuroprotective effect of C3 transferase in terms of reduced p75NTR-mediated apoptosis (Dubreuil et al. 2003) and preserved white or gray matter tissue has been demonstrated (Boato et al. 2010; Lord-Fontaine et al. 2008). C3 transferase has been delivered in various ways. It has been injected intramedullarly rostral to the lesion (Schwab et al. 2002) or applied at the lesion site within a fibrin clot either intrathecally (Dubreuil et al. 2003) or extradurally (Boato et al. 2010; Lord-Fontaine et al. 2008). The effects of direct RhoA-inhibition after SCI have recently been verified by the suppression of RhoA expression through short interfering RNA (siRNA). The intrathecal application of siRNA targeted against RhoA led to enhanced serotonergic sprouting, improved locomotion and spared white matter tissue in an SCI contusion model (Otsuka et al. 2011). Furthermore, attenuated allodynia was first demonstrated in this model after RhoA-suppression following experimental SCI (Otsuka et al. 2011), whereas Lord-Fontaine et al. 2008 did not observe an effect on allodynia after Rho-inhibition. However, in a diabetic mouse model of neuropathic pain, intrathecal C3 transferase led to reduced hyperalgesia (Ohsawa et al. 2011); C3 transferase pretreatment abolished allodynia and hyperalgesia induced by intrathekal lysophosphatic acid (LPA) injection and also by peripheral injury to the sciatic nerve (Inoue et al. 2004).
ROCK-inhibition
Experimental blockade through the ROCK-inhibitor Y-27632 yields results comparable with direct Rho-inhibition, whether in vitro (Boato et al. 2010; Borisoff et al. 2003; Dergham et al. 2002; Fournier et al. 2003; Julien et al. 2008; Monnier et al. 2003; Tanaka et al. 2004) or in vivo. Here, intrathecal delivery of Y-27632 promotes CST sprouting and the recovery of locomotion after transectional SCI (Chan et al. 2005; Dergham et al. 2002; Fournier et al. 2003). A reduction of scar tissue (Fournier et al. 2003), lesion cavity area (Tanaka et al. 2004) and a retardation of Wallerian degeneration of CST axons (Yamagishi et al. 2005) have been observed. The findings of the Y-27632 experiments are supported by ROCK-inhibition through fasudil (HA-1077), the protein p21CIP1/WAF1 (Tanaka et al. 2004), or lentiviral transduction with dominant negative mutant ROCK (Wu et al. 2009), which revealed CST (Tanaka et al. 2004) or rubrospinal tract (Wu et al. 2009) axonal sprouting responses and improved functional recovery attributable to the treatment after transectional SCI. Most convincingly, genetic deletion of the ROCK isoform ROCK-II permits substantial axonal sprouting and regeneration from multiple fiber systems after CNS injury (Duffy et al. 2009).
The neuroprotecting effect reducing secondary damage after ischemic CNS injury through ROCK-blockade has been verified after intraperitoneal Y-27632 or fasudil application in models of experimental stroke (Li et al. 2009; Rikitake et al. 2005; Satoh et al. 1996, 2008; Shin et al. 2007; Toshima et al. 2000). Additionally, fasudil protects neurological function (Li et al. 2009; Satoh et al. 1996, 2008; Toshima et al. 2000; Yamashita et al. 2007). The tissue sparing was attributable to enhanced bloodflow related to increased endothelial nitric oxide synthase activity (Li et al. 2009; Rikitake et al. 2005; Satoh et al. 2008; Shin et al. 2007). Fasudil has also been reported to protect neurons from cell death after hypoxia (Ding et al. 2010) and acute (Yamashita et al. 2007) and chronic (Huang et al. 2008) ischemia. This is associated with the suppression of the microglial response and attenuated production of pro-inflammatory cytokines such as interleukin-1β and TNF-α in vivo (Ding et al. 2010) or to the restricted infiltration of neutrophils (Satoh et al. 2008). Additionally, the protein expression of inducible nitric oxide synthase was reduced (Ding et al. 2010; Li et al. 2009). Further potential targets of ROCK-inhibiting therapeutics are atherosclerosis and cardiovascular diseases, as reviewed elsewhere (Zhou et al. 2011).
Beneficial effects of ROCK-inhibition within the CNS on the development of neuropathic pain are widely similar to those of the direct Rho-blockade as demonstrated in the neuropathic pain models in diabetic mice (Ohsawa et al. 2011) and the LPA or sciatic nerve injury model (Inoue et al. 2004) in which Y-27632 has been compared with C3 transferase. These studies have been complemented by further peripheral nerve injury experiments. After rhizotomy to the dorsal root, Y-27632 attenuates cold hyperalgesia (Ramer et al. 2004). Intrathecal treatment with the more selective ROCK-inhibitor dimethylfasudil (H-1152) diminishes mechanical allodynia after lumbar nerve transection (Tatsumi et al. 2005).
Small-molecule-mediated Rho-inhibition
NSAIDs are widely used as analgesic and anti-inflammatory drugs. Their mechanism of action as an analgesic and anti-phlogistic/anti-rheumatic agent consists in the inhibition of prostaglandin synthesis through the unselective inhibition of COX-1 and COX-2. Furthermore, NSAIDs inhibit ADP-and collagen-induced platelet aggregation in a reversible manner (Schafer 1999). Independently from COX-inhibition, individual NSAIDs inhibit basal (Dill et al. 2010; Fu et al. 2007; Wang et al. 2009; Zhou et al. 2003) and LPA-receptor-mediated (Fu et al. 2007; Wang et al. 2009) RhoA-activation.
In vitro, the ability to reduce levels of activated RhoA has been concordantly demonstrated for ibuprofen (Dill et al. 2010; Fu et al. 2007; Wang et al. 2009; Zhou et al. 2003). For indomethacin (Fu et al. 2007; Zhou et al. 2003) and suldinac sulfide (Zhou et al. 2003), a Rho-inhibitory effect has also been reported but has not been confirmed by others (Wang et al. 2009). Additional investigated NSAIDs, i.e., naproxen, piroxicam, meloxicam and SC-560, do not block the activation of Rho (Wang et al. 2009; Zhou et al. 2003). Whereas the inhibition of COX occurs nearly exclusively via the S(+)-enantiomer (Davies 1998), blockage of RhoA-activation has been shown for both enantiomers of ibuprofen (Wang et al. 2009; Zhou et al. 2003). The amount of activated RhoA in cultured neurons plated on axon-growth inhibitory substrates such as CSPGs and myelin/MAG is reduced by ibuprofen and indomethacin application, similar to the specific RhoA-inhibitor C3 transferase (Fu et al. 2007).
Consequently, ibuprofen prevents the MAG-induced collapse of axonal growth cones similar to the ROCK-inhibitor Y-27632 but does not prevent Sema3A-operated growth cone collapse, which occurs independently from the Rho pathway (Wang et al. 2009). Enhanced axonal sprouting in the presence of CSPGs and myelin/MAG in the culture of neuronal cells has been observed following treatment with ibuprofen (Dill et al. 2010; Fu et al. 2007; Wang et al. 2009) and with indomethacin (Fu et al. 2007) but not with naproxen. The extent of the growth-promoting effect of ibuprofen in vitro conforms to that of C3 transferase and Y-27632 (Fu et al. 2007; Wang et al. 2009). Prevention of oligodendrocyte cell death after TNF-α pretreatment has been recorded in the presence of ibuprofen or indomethacin similarly to C3 transferase. Again, naproxen has no effect on apoptosis (Xing et al. 2011). Ibuprofen-mediated RhoA-inhibition is linked to the peroxisome proliferator activated receptor γ (PPARγ), as demonstrated in vitro by using the PPARγ antagonist GW9662 and by PPARγ knockdown with siRNA, both of which abolish ibuprofen-induced RhoA-inhibition (Dill et al. 2010).
In vivo Rho-inhibition and plasticity
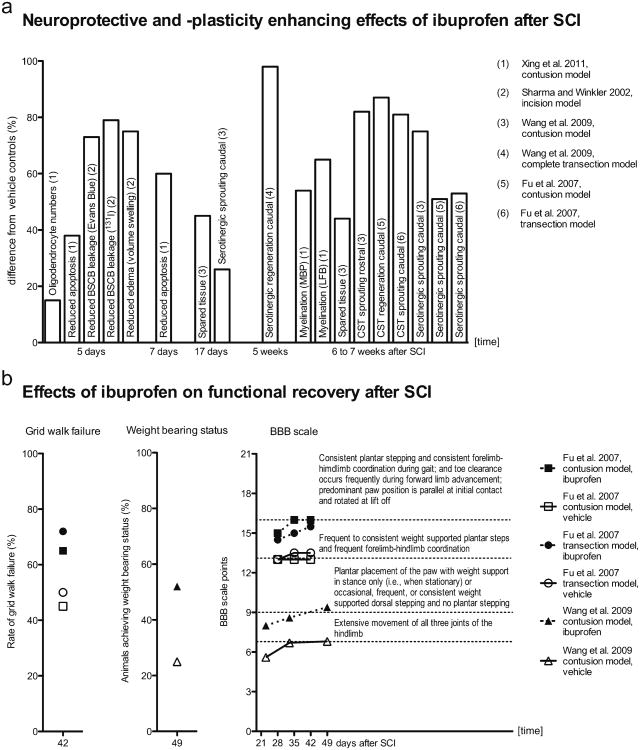
RhoA activity assays have provided independent confirmation of the Rho-inhibiting effect of ibuprofen after SCI as shown by Fu et al. (2007) and Wang et al. (2009). Axonal sprouting and the density of nerve fibers were registered in both studies as non-functional surrogate parameters of the regenerative efficacy of Rho-inhibition as mediated by systemically administered ibuprofen compared with vehicle alone or naproxen. The compounds were applied subcutaneously over 4 weeks, starting within 1 week post-SCI. A greater density of CST fibers with a higher degree of sprouting axons rostral to the lesion after rat spinal cord contusion (Fu et al. 2007; Wang et al. 2009) and transection (Fu et al. 2007) was reported. In contrast to Wang et al. (2009), Fu et al. (2007) found increased axonal sprouting in the CST, even caudal to the lesion in the contusion and transection model. Both studies reported a higher density of serotonergic raphespinal fibers, mainly rostral to but to a lesser extent also distal to, the injury site after administration of ibuprofen. After complete transection of the spinal cord in a mouse model, animals treated with ibuprofen had serotonergic fibers growing caudally through the lesion. No serotonergic fibers were seen caudal to the lesion in the control group. A higher dose of ibuprofen was associated with greater efficacy (Wang et al. 2009).
In summary, ibuprofen triggers axonal plasticity of the corticospinal and the raphespinal tract (Table 1, Fig. 2a). This is in agreement with trials on specific Rho-blockade (Boato et al. 2010; Dergham et al. 2002) and ROCK-inhibition (Chan et al. 2005; Dergham et al. 2002; Fournier et al. 2003; Tanaka et al. 2004; Wu et al. 2009).
Table 1.
Ibuprofen-mediated RhoA-inhibition after experimental spinal cord injury (BBB Basso-Beattie-Bresnahan scale from Basso et al. 1995, BSCB blood-spinal-cord-barrier, BMS Basso Mouse Scale from Basso et al. 2006, CST corticospinal tract, n.s. not significant, n.t. not tested, OLG oligodendrocytes, SCI spinal cord injury, SCEP spinalcord-evoked potentials)
| Author | SCI model | Treatment period | Follow-up period | Functional improvement | Non-functional improvement | Rho- inhibition |
|---|---|---|---|---|---|---|
| Xing et al. 2011 | T8/9 contusion (rat) | 1 h to day 5 | Day 5 | n.t. | Reduced apoptosis Increased OLG numbers | n.t. |
| 1 h to day 7 | Day 7 | Reduced apoptosis | ||||
| 1 h to day 28 | Day 28 or day 42 | Enhanced myelination | ||||
| Wang et al. 2009 | T7 contusion (rat) | day 1 to day 31 | 49 days | BBB | Spared tissue CST-sprouting Serotinergic-sprouting | Immuno-blot |
| T8 complete transection (mouse) | day 1 to day 28 | 35 days | n.s. (BMS) | Serotinergic-regeneration | n.t. | |
| Fu et al. 2007 | T8/9 contusion (rat) | day 7 to day 35 | 42 days | BBB Grid-walk-failure | CST-regeneration Serotinergic-sprouting | Immuno-blot |
| T6/7 hemisection (rat) | day 1 to day 28 | 42 days | BBB Grid-walk-failure | CST-sprouting Serotinergic-sprouting | n.t. | |
| Sharma and Winkler 2002 | T11 incision (rat) | 30 min prior | 5h | n.t. | Spinal-cord edema SCEP BCSB-permeability | n.t. |
Fig. 2.
Neuroprotective, plasticity-enhancing and functional neurological improving effects of ibuprofen-mediated RhoA-inhibition after experimental spinal cord injury (SCI). Survey of statistically significant observations (depicted from: Xing et al. 2011; Sharma and Winkler 2002; Wang et al. 2009; Fu et al. 2007). a Differences in cell survival, tissue protection and sprouting responses in ibuprofen-treated animals relative to vehicle controls were calculated from the published original data. Neuroprotective effects were detectable within the first week after SCI. Enhanced myelination and spouting of distinct fiber tracts were observed several weeks after SCI. Because the effects on apoptosis and myelination (Xing et al. 2011) were principally similar for areas caudal and rostral to the injury site, average values from caudal and rostral areas are presented here (BSCB blood-spinal-cord-barrier, CST corticospinal tract, MBP myelin basic protein, LFB Luxol fast blue). b Results of behavioral testing in the ibuprofen and vehicle groups. The BBB scale (Basso-Beattie-Bresnahan scale, Basso et al. 1995) differences are expressed as absolute scale categories and the respective categories are explained in the graph
Trials aiming specifically to evaluate indomethacin in vivo as a putative Rho-inhibitor have not as yet been undertaken. Nevertheless, indomethacin has been investigated with regard to lesional RhoA-expression after SCI with respect to its COX-inhibitory properties (Schwab et al. 2004). Here, a reduction of RhoA-positive lesional cell numbers, mainly confined to granulocytes and microglia/macrophages, has been demonstrated after intraperitoneal injection of indomethacin for 3 days after spinal cord transection.
Functional recovery
In rat spinal cord contusion and transection studies, residual neurological function has been investigated under ibuprofen treatment. After subcutaneous systemic administration of ibuprofen, the open-field BBB scale (Basso et al. 1995) improves (Fu et al. 2007; Wang et al. 2009) and grid walk failure is diminished (Fu et al. 2007) compared with vehicle controls and the non-Rho-inhibiting NSAID naproxen. In particular, a larger proportion of animals achieve weightbearing status (Wang et al. 2009). The improvement in locomotor function has been independently reproduced within therapeutic time-frames from day 1 to 28, day 3 to 31 (Fu et al. 2007; Wang et al. 2009) and day 7 to 35 (Fu et al. 2007) after SCI. The treatment effect becomes visible 3–4 weeks after SCI and is present until up to 7 weeks after SCI (Fu et al. 2007; Wang et al. 2009). Thus, the effect is robust and persists after the end of the administration of the active substance. The results of behavioral testing under ibuprofen treatment after experimental spinal cord contusion and transection in the rat model (Table 1, Fig. 2b) are consistent with the locomotor improvement reported for C3 transferase treatment in corresponding SCI models (Dergham et al. 2002; Lord-Fontaine et al. 2008; Schwab et al. 2002). The recovery of neurological function in the mouse model of complete spinal cord transection is mild as expected (Wang et al. 2009).
Besides ibuprofen mostly indomethacin has primarily been evaluated with regard to locomotor function after SCI. Following experimental spinal cord contusion in the rat, systemic treatment starting immediately after injury leads to improved locomotion. The improvement, as measured by the Tarlov motor score or its modifications (Wrathall et al. 1985), is not detectable at day 1 but rather at 6 weeks post-trauma compared with vehicle (Simpson et al. 1991). Conversely, no significant changes in the motor score have been observed at 4 weeks after SCI in indomethacin-treated rats after spinal cord compression (Guth et al. 1994). In a rabbit model of spinal cord contusion, animals treated daily with indomethacin starting immediately after injury have been reported to have a better motor score function visible from day 1 to the end of the observation at day 9 after SCI (Pantovic et al. 2005). These early treatment effects on motor function have not been observed in the rodent trials published for NSAID treatment after SCI. However, for Rho-inhibition through C3 transferase in rat or mouse SCI models, early persistent improvement is visible in locomotion at day 1 or 2 post-injury as measured by the BBB scale or BMS, respectively (Boato et al. 2010; Dergham et al. 2002).
Neuroprotection
Spared tissue has been seen at the lesion site after ibuprofen treatment following spinal cord contusion (Wang et al. 2009). This is in agreement with observations after RhoA down-regulation (Otsuka et al. 2011), C3 transferase (Boato et al. 2010; Lord-Fontaine et al. 2008), or Y-27632 (Tanaka et al. 2004) treatments. In addition, the neuroprotective effect of ibuprofen following SCI has been demonstrated in a dorsal horn incision model in the rat leading to significant recovery of alterations in spinal-cord-evoked potentials and to a reduction of spinal cord edema and blood-spinal-cord-barrier damage following SCI (Sharma and Winkler 2002). Ibuprofen administration for 5 or 7 days, starting 1 h after rat spinal cord contusion, results in lower numbers of apoptotic cells and slightly higher numbers of oligodendrocytes caudal and rostral to the spinal cord lesion site. Furthermore, enhanced myelination has been reported after treatment for 28 days (Xing et al. 2011; Table 1, Fig. 2a). The activation of PPARγ through ibuprofen (Dill et al. 2010) further explains its neuroprotective properties, since PPARγ activation has repeatedly been demonstrated to have beneficial effects, for example, on cell survival and axonal myelination following SCI (for a review, see McTigue 2008).
Similar neuroprotective effects as those seen after ibuprofen treatment were reported after high-dose indomethacin pretreatment by Sharma and colleagues in the incision model (Sharma and Winkler 2002; Sharma et al. 1993a, b; Winkler et al. 1993). Attenuation of histopathological changes after post-injury delivery of indomethacin were also observed by Simpson et al. (1991). Guth et al. (1994) were unable to report a tissue-protective effect with regard to a reduction of the lesion cavity but notably, here, indomethacin was applied at a low dosage that also yielded no protective effect on spinal cord edema elsewhere (Sharma et al. 1993a). Indomethacin treatment in the study of Xing et al. (2011) yielded effects comparable with those of ibuprofen with regard to a reduction of apoptotic cell numbers and protection of oligodendrocytes. Also, the promotion of myelination was also demonstrated after indomethacin administration but to a lesser degree (Xing et al. 2011).
Neuroprotective properties of NSAIDs have been additionally explored in models of neurodegenerative disease. Ibuprofen (Lim et al. 2001; Weggen et al. 2001, 2003; Zhou et al. 2003), indomethacin and suldinac sulfide lower Aβ42, whereas NSAIDs without significant Rho-inhibiting properties are not effective. Consequently, a reduction of Aβ42 peptide levels has been confirmed to be independent of COX-inhibition (Weggen et al. 2001) but is linked to the inhibition of Rho (Zhou et al. 2003).
RhoA-inhibition and classical NSAID-operated effects
To summarize the cumulative evidence for ibuprofen-mediated Rho-inhibition, the main neurobiological effect involves the enhanced axonal plasticity in the spinal segments directly adjacent to the lesion site. Rostral and caudal to the lesion site, axonal sprouting might lead to a reorganization of spinal circuits and reconnections within spinal tracts from injured axons to preserved axons (Curt et al. 2008). On the other hand, no concordant preclinical evidence has been demonstrated for the regeneration of nerve fibers tracts far beyond the lesion. Thus, long-distance regeneration of spinal tracts is less likely to occur.
Next, the neuroprotective effects of Rho-inhibition might lead to a lower degree of secondary damage. Additional favorable effects of Rho-inhibition through small molecules might also occur because of their “classical” mode of action, i.e., the reduction of COX-mediated inflammatory responses in the region of the secondary damage (Schwab et al. 2004). In addition, the activation of PPARγ also has anti-inflammatory effects and exerts tissue protection (McTigue 2008). These additional effects of non-selective Rho-inhibition might antagonize some possibly adverse effects of selective Rho/ROCK-inhibition. Although the diminution of inflammatory responses has been reported after ROCK-inhibition (Ding et al. 2010; Li et al. 2009; Satoh et al. 2008), contradictory data also exist. More precisely, a specific block of Rho-activation by C3 transferase seems to cause, in microglia, a pro-inflammatory profile that is partly controlled by nuclear factor kappa B (NF-κB; Hoffmann et al. 2008). In addition, ROCK-inhibition through Y-27632 ia associated with pronounced astrocyte activation and increased production of CSPGs after SCI (Chan et al. 2007). Thus, a high-dose ibuprofen application might mitigate proinflammatory components of specific Rho-inhibiton. Together, NSAID-operated COX- and NF-κB-inhibition (Scheuren et al. 1998) and PPARγ-activation (Dill et al. 2010) might account for a reduction of the inflammatory milieu that triggers progliotic effects.
Neuropathic pain is a common and persisting consequences of SCI (Siddall and Loeser 2001; Siddall et al. 1999). With regard to therapies aiming to promote neuronal plasticity/regeneration in patients with lesions of the CNS, inappropriate synaptic connections may unfortunately give rise to neuropathic pain syndromes. In some of the clinical investigations of neurotrophins in patients with degenerative diseases of the CNS, radicular pain and headache are reported as side effects (Thoenen and Sendtner 2002). In contrast, a growing body of preclinical evidence indicates that Rho-inhibition has beneficial effects on neuropathic pain (Inoue et al. 2004; Ohsawa et al. 2011; Ramer et al. 2004; Tatsumi et al. 2005). In addition to Rho-inhibition, NSAIDs might exert further mechanisms that attenuate factors responsible for the emergence of central pain (Detloff et al. 2008; Milligan and Watkins 2009). The anti-phlogistic effects of NSAIDs might enhance tissue protection, reduce glial scar tissue and attenuate further inflammatory stimuli of pain syndromes. For example, one of the factors believed to be involved in the emergence of neuropathic pain is the up-regulation of the P2X4 receptor (Tsuda et al. 2003). Fibronectin as a component of the ECM within the glial scar tissue promotes P2X4 expression and the emergence of pain (Tsuda et al. 2008). The P2X4 receptor is up-regulated after injury to peripheral nerves (Tsuda et al. 2003) and after SCI (Schwab et al. 2005). In addition, after experimental peripheral inflammation, P2X4 is also up-regulated in the CNS (Guo et al. 2005). Thus, even the reduction of peripheral inflammation through high-dose ibuprofen or indomethacin treatment is likely to attenuate mechanisms involved in the development of central pain.
Translational aspects
The Rho-pathway is a major target for plasticity-enhancing therapy after damage to the CNS (McKerracher and Higuchi 2006; Mueller et al. 2005; Rossignol et al. 2007). From the group of Rho-inhibiting NSAIDs, ibuprofen is constantly confirmed to inhibit RhoA in vitro and in vivo. Noteworthy, the promoting effect on axonal plasticity/regeneration (Fig. 2a) and functional recovery (Fig. 2b) is in agreement with the evidence from C3 transferase and Y-27632 studies.
The animal models used, namely spinal cord transection and contusion, are internationally accepted (Kwon et al. 2002). The rat SCI-contusion model is comparable with SCI after trauma to the vertebral column in humans (Metz et al. 2000a) and is recommended for preclinical investigations (Tator 2006). The standardized transection model that has been used in rats is feasible for studying signs of neuroanatomical and neurofunctional recovery as reported earlier (Merkler et al. 2001). The measurement of functional outcome after ibuprofen treatment has been performed by using recent and validated instruments such as the widely used BBB score, grid walk failure and footprint analysis (Metz et al. 2000b). A weakness of the study of Fu et al. (2007) is that the functional outcome has been statistically evaluated by using Student's t-test. Since the BBB scale is nonlinear, analysis with nonparametric tests or contingency table evaluation, as performed by Wang et al. (2009), appears more appropriate.
Indomethacin has been identified as a further Rho-inhibiting NSAID but this has not been reproduced consistently. However, enhanced axonal outgrowth on myelin and CSPG has been demonstrated in vitro. The publications from in vivo trials focus on the effects of indomethacin as a COX-inhibitor and do not present investigations of specific axonal sprouting responses but report tissue protective effects. Evidence for improved functional recovery after experimental spinal cord contusion has been demonstrated in the rat and reproduced in the rabbit model. Functional outcome in the indomethacin trials has been assessed by using the Tarlov scale, which represents an earlier, less sensitive measurement tool compared with the BBB scale (Metz et al. 2000b).
In summary, ibuprofen constitutes the best-investigated Rho-inhibiting NSAID at present. It has been evaluated in recent animal studies with regard to Rho-inhibition within a feasible time-frame of therapeutic opportunity. A refined setting of non-functional and behavioral assessment has been applied as efficacy measurements in clinically relevant models of SCI (Table 1, Fig. 2).
CNS permeability
CNS uptake of ibuprofen seems adequate, since systemically applied ibuprofen leads to substantial Rho-inhibition in spinal cord tissue (Fu et al. 2007; Wang et al. 2009). The bioactive unbound ibuprofen fraction passes the blood-brain barrier unhindered. Experimental investigations of perfusion models in animals have shown rapid linear absorption of radioactivelabeled ibuprofen and other NSAIDs. However, albuminbound ibuprofen does not pass the blood-brain barrier (Mandula et al. 2006; Parepally et al. 2006). Thus, differences in albumin binding should be taken into account when calculating the human equivalence dose (HED). Ibuprofen has a higher affinity for binding to human albumin compared with that to rat albumin. Additionally, rat serum has a slightly lower albumin concentration. Hence, the free fraction of ibuprofen is two- to three-fold higher in rat serum compared with human serum (Mandula et al. 2006; Mills et al. 1973). Ibuprofen doses of 60 or 70 mg/kg per day have been used in rat SCI models (Fu et al. 2007; Wang et al. 2009). The pharmacologically active dose (PAD) in humans can be calculated based on body surface by using a conversion model that is feasible for systemically administered active substances of a small molecular size (Center for Drug Evaluation and Research, FDA 2005). The HEDs derived from this model are 9.7 or 11.3 mg/kg per day. For an estimation of the PAD with respect to the above-mentioned differences in albumin binding, the HED should be multiplied by a factor of approximately 3. Nevertheless, a PAD of 30 mg/kg per day is within the FDA-approved range for application in humans.
Implications for clinical use
Blockage of the Rho-pathway has aroused international interest in the field of clinical translational research. Interventional trials on intrathecally administered anti-Nogo-A antibodies (http://clinicaltrials.gov/ct2/show/NCT00406016) and the neurosurgically applied C3-transferase preparation BA-210, also known as Cethrin (Fehlings et al. 2011) address this growth inhibitory signal cascade at the ligand level or “downstream” through direct RhoA-inhibition, respectively. BA-210 has previously been investigated in a phase I/IIa trial in North America (http://clinicaltrials.gov/ct2/show/NCT00500812). The data obtained from 48 patients indicated the safety of the Rho-inhibition-based intervention and exploratory data are suggestive of an improvement of residual neurological function (Fehlings et al. 2011).
The ROCK-inhibitor fasudil is commonly used in Japan for the prevention of vasospasms after subarachnoid hemorrhage. Fasudil has also been investigated in indications of ischemic stroke within a randomized controlled trial that enrolled 160 patients in Japan. The investigators reported a better neurological status, without severe adverse reactions, in the fasudil group (Shibuya et al. 2005).
Based on the evidence for ibuprofen-mediated Rho-inhibition, better recovery of neurological function in patients suffering from acute traumatic SCI can be anticipated after the administration of ibuprofen. Further therapeutic targets of the compound might accomplish the required plasticity-promoting effects. The analgesic effect of ibuprofen might be expected to reduce nociceptive pain for the duration of the intervention and additional anti-inflammatory effects of the compound might attenuate the development of the neuroathic pain that is a frequent and chronic complication after SCI (Siddall and Loeser 2001; Siddall et al. 1999). Moreover, ibuprofen might prevent heterotopic ossifications that are also frequent complications after SCI. Indomethacin treatment in an anti-inflammatory dosage has previously proven to be effective in this regard (van Kuijk et al. 2008).
Ibuprofen is an approved drug, risk profile and pharmacological properties are well known (Davies 1998). The risks of long-term use of NSAID are primarily gastrointestinal ulcers and hemorrhage and, more rarely, acute renal failure during treatment. However, within the group of NSAIDs, ibuprofen has a lower toxicity because of its relatively short half-life (Sing and Ramey 1998; Whelton 1999). Additionally, long-term high-dose ibuprofen use as an “orphan drug” in the indication of cystic fibrosis has been assessed as having a positive risk-benefit profile (Konstan 2008; Lands and Stanojevic 2007). In the setting of acute care of SCI patients, risk factors can be controlled, by close in-hospital monitoring.
Concluding remarks
In a large number of patients, traumatic and complete SCI signifies a severe lifelong physical disability that poses a challenge for the patient's occupational and social integration. The only standard treatment to promote functional recovery after SCI is physiotherapy. Based on preclinical investigations in established animal models, enhanced neu-ronal plasticity/regeneration and better recovery of neurological function is anticipated from the use of the Rho-inhibitor ibuprofen in cases of acute SCI. This might lead to a marked improvement of vital functions and aspects of daily living, even if the plasticity-based recovery affects only one or two segments of the spinal cord. The small-molecule-operated Rho-inhibition provides the opportunity to investigate clinically an available, long-established, globally used medication with established pharmacological properties. Compared with BA-210 and antibodies to Nogo-A, ibuprofen can be easily administered by the oral route and has a well-known and acceptable long-term risk profile. Additionally, the dosage and time-frame of the intervention are feasible for clinical application. This results in an ethically favourable “benefit-risk ratio”. Assuming therapeutic equivalence, the clinical application of small-molecule-mediated Rho-inhibition would not only be meaningful in economic terms compared with recently developed approaches but also might hold promise to improve health by facilitating neurofunctional rehabilitation and exert further preventive effects on serious complications after traumatic SCI.
Acknowledgments
This work is funded by the German Research Council (DFG, Research Training School, Neuroinflammation No. 1258 & Exc 247), German Ministry of Science and Education, the Berlin-Brandenburg Center for Regenerative Therapies (BCRT, No. 81717034), the International Foundation for Research in Paraplegia, Switzerland (IFP, no. P102) and Wings for Life Spinal Cord Research Foundation, Austria (no. 89830429). Spinal cord injury Reaserch, Department of Experimental Neurology, Charité is Associated Member of the European Multi-center Study about Spinal Cord Injury (EMSCI).
Footnotes
Author disclosure statement: S.M.S. is a co-founder of Axerion Therapeutics seeking to develop NgR- and PrP-based therapies.
Contributor Information
M. A. Kopp, Email: jan.schwab@charite.de, Department of Neurology and Experimental Neurology, Spinal Cord Injury Research, Charité—Universitätsmedizin Berlin, Charitéplatz 1, 10117 Berlin, Germany.
T. Liebscher, Trauma Hospital Berlin, Treatment Centre for Spinal Cord Injuries, Berlin, Germany
A. Niedeggen, Trauma Hospital Berlin, Treatment Centre for Spinal Cord Injuries, Berlin, Germany
S. Laufer, Department of Pharmaceutical and Medicinal Chemistry, Institute of Pharmacy, Eberhard Karls University Tübingen, Tübingen, Germany
B. Brommer, Email: jan.schwab@charite.de, Department of Neurology and Experimental Neurology, Spinal Cord Injury Research, Charité—Universitätsmedizin Berlin, Charitéplatz 1, 10117 Berlin, Germany.
G. J. Jungehulsing, Department of Neurology and Experimental Neurology, Center for Stroke Research Berlin, Charité—Universitätsmedizin Berlin, Berlin, Germany
S. M. Strittmatter, Program in Cellular Neuroscience, Neurodegeneration and Repair, Department of Neurology, Yale University School of Medicine, New Haven, USA
U. Dirnagl, Department of Neurology and Experimental Neurology, Center for Stroke Research Berlin, Charité—Universitätsmedizin Berlin, Berlin, Germany
J. M. Schwab, Email: jan.schwab@charite.de, Department of Neurology and Experimental Neurology, Spinal Cord Injury Research, Charité—Universitätsmedizin Berlin, Charitéplatz 1, 10117 Berlin, Germany.
References
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Barton WA, Liu BP, Tzvetkova D, Jeffrey PD, Fournier AE, Sah D, Cate R, Strittmatter SM, Nikolov DB. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J. 2003;22:3291–3302. doi: 10.1093/emboj/cdg325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus K, James ND, Bosch KD, Bradbury EJ. Chondroitin sulphate proteoglycans: key modulators of spinal cord and brain plasticity. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci USA. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boato F, Hendrix S, Huelsenbeck SC, Hofmann F, Grosse G, Djalali S, Klimaschewski L, Auer M, Just I, Ahnert-Hilger G, Höltje M. C3 peptide enhances recovery from spinal cord injury by improved regenerative growth of descending fiber tracts. J Cell Sci. 2010;123:1652–1662. doi: 10.1242/jcs.066050. [DOI] [PubMed] [Google Scholar]
- Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, Steeves JD, Tetzlaff W. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci. 2003;22:405–416. doi: 10.1016/s1044-7431(02)00032-5. [DOI] [PubMed] [Google Scholar]
- Buchli AD, Schwab ME. Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann Med. 2005;37:556–567. doi: 10.1080/07853890500407520. [DOI] [PubMed] [Google Scholar]
- Center for Drug Evaluationand Research, FDA. Guidance for industry, estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult health volunteers. 2005 www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf.
- Chacon PJ, Garcia-Mejias R, Rodriguez-Tebar A. Inhibition of RhoA GTPase and the subsequent activation of PTP1B protects cultured hippocampal neurons against amyloid β toxicity. Mol Neurodegener. 2011;6:14. doi: 10.1186/1750-1326-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Khodarahmi K, Liu J, Sutherland D, Oschipok LW, Steeves JD, Tetzlaff W. Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp Neurol. 2005;196:352–364. doi: 10.1016/j.expneurol.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Chan CC, Wong AK, Liu J, Steeves JD, Tetzlaff W. ROCK inhibition with Y27632 activates astrocytes and increases their expression of neurite growth-inhibitory chondroitin sulfate proteoglycans. Glia. 2007;55:369–384. doi: 10.1002/glia.20466. [DOI] [PubMed] [Google Scholar]
- Conrad S, Genth H, Hofmann F, Just I, Skutella T. Neogenin-RGMa signaling at the growth cone is bone morphogenetic protein-independent and involves RhoA, ROCK, and PKC. J Biol Chem. 2007;282:16423–16433. doi: 10.1074/jbc.M610901200. [DOI] [PubMed] [Google Scholar]
- Curt A, Hedel HJ van, Klaus D, Dietz V, EM-SCI Study Group Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma. 2008;25:677–685. doi: 10.1089/neu.2007.0468. [DOI] [PubMed] [Google Scholar]
- Davies NM. Clinical pharmacokinetics of iburofen. The first 30 years. Clin Pharmacokinet. 1998;34:101–154. doi: 10.2165/00003088-199834020-00002. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and proinflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. 2008;212:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill J, Patel AR, Yang XL, Bachoo R, Powell CM, Li S. A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. J Neurosci. 2010;30:963–972. doi: 10.1523/JNEUROSCI.5045-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Li QY, Wang X, Sun CH, Lu CZ, Xiao BG. Fasudil protects hippocampal neurons against hypoxia-reoxygenation injury by suppressing microglial inflammatory responses in mice. J Neurochem. 2010;114:1619–1629. doi: 10.1111/j.1471-4159.2010.06876.x. [DOI] [PubMed] [Google Scholar]
- Dubreuil CI, Winton MJ, McKerracher L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J Cell Biol. 2003;162:233–243. doi: 10.1083/jcb.200301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy P, Schmandke A, Sigworth J, Narumiya S, Cafferty WB, Strittmatter SM. Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J Neurosci. 2009;29:15266–15276. doi: 10.1523/JNEUROSCI.4650-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlings MG, Theodore N, Harrop J, Maurais G, Kuntz C, Shaffrey CI, Kwon BK, Chapman J, Yee A, Tighe A, McKerracher L. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma. 2011;28:787–796. doi: 10.1089/neu.2011.1765. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Gould GC, Liu BP, Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002;22:8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007;27:4154–4164. doi: 10.1523/JNEUROSCI.4353-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Endo S, Takai T, Yamashita T. Myelin suppresses axon regeneration by PIR-B/SHP-mediated inhibition of Trk activity. EMBO J. 2011;30:1389–1401. doi: 10.1038/emboj.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Spanevello MD, Tajouri S, Li L, Rogers F, Pearse M, Galea M, Bartlett PF, Boyd AW, Turnley AM. EphA4 blockers promote axonal regeneration and functional recovery following spinal cord injury in mice. PLoS One. 2011;6:e24636. doi: 10.1371/journal.pone.0024636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPré T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Guo LH, Trautmann K, Schluesener HJ. Expression of P2X4 receptor by lesional activated microglia during formalin-induced inflammatory pain. J Neuroimmunol. 2005;163:120–127. doi: 10.1016/j.jneuroim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Guth L, Zhang Z, DiProspero NA, Joubin K, Fitch MT. Spinal cord injury in the rat: treatment with bacterial lipopolysaccharide and indomethacin enhances cellular repair and locomotor function. Exp Neurol. 1994;126:76–87. doi: 10.1006/exnr.1994.1043. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Hofmann F, Just I, Lehnardt S, Hanisch UK, Brück W, Kettenmann H, Ahnert-Hilger G, Höltje M. Inhibition of Rho-dependent pathways by Clostridium botulinum C3 protein induces a proinflammatory profile in microglia. Glia. 2008;56:1162–1175. doi: 10.1002/glia.20687. [DOI] [PubMed] [Google Scholar]
- Hu F, Strittmatter SM. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci. 2008;28:1262–1269. doi: 10.1523/JNEUROSCI.1068-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, He Z, Guo L, Wang H. Improvement of cognitive deficit and neuronal damage in rats with chronic cerebral ischemia via relative long-term inhibition of rho-kinase. Cell Mol Neurobiol. 2008;28:757–768. doi: 10.1007/s10571-007-9157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner EA, Kim BG, Duffy PJ, Brown RH, Strittmatter SM. A multi-domain fragment of Nogo-A protein is a potent inhibitor of cortical axon regeneration via Nogo receptor 1. J Biol Chem. 2011;286:18026–18036. doi: 10.1074/jbc.M110.208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10:712–718. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien S, Schnichels S, Teng H, Tassew N, Henke-Fahle S, Mueller BK, Monnier PP. Purkinje cell survival in organotypic cultures: implication of Rho and its downstream effector ROCK. J Neurosci Res. 2008;86:531–536. doi: 10.1002/jnr.21511. [DOI] [PubMed] [Google Scholar]
- Konstan MW. Ibuprofen therapy for cystic fibrosis lung disease: revisited. Curr Opin Pulm Med. 2008;14:567–573. doi: 10.1097/MCP.0b013e32831311e8. [DOI] [PubMed] [Google Scholar]
- Kottis V, Thibault P, Mikol D, Xiao ZC, Zhang R, Dergham P, Braun PE. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J Neurochem. 2002;82:1566–1569. doi: 10.1046/j.1471-4159.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- Kuijk AA van, Geurts AC, Kuppevelt HJ van. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord. 2008;40:313–326. doi: 10.1038/sj.sc.3101309. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Oxland TR, Tetzlaff W. Animal models used in spinal cord regeneration research. Spine. 2002;27:1504–1510. doi: 10.1097/00007632-200207150-00005. [DOI] [PubMed] [Google Scholar]
- Lands LC, Stanojevic S. Oral non-steroidal anti-inflammatory drug therapy for cystic fibrosis. Cochrane Database Syst Rev. 2007;4:CD001505. doi: 10.1002/14651858.CD001505.pub2. [DOI] [PubMed] [Google Scholar]
- Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu BP, Budel S, Li M, Ji B, Walus L, Li W, Jirik A, Rabacchi S, Choi E, Worley D, Sah DW, Pepinsky B, Lee D, Relton J, Strittmatter SM. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Huang XJ, He W, Ding J, Jia JT, Fu G, Wang HX, Guo LJ. Neuroprotective potential of fasudil mesylate in brain ischemiareperfusion injury of rats. Cell Mol Neurobiol. 2009;29:169–180. doi: 10.1007/s10571-008-9308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, Hamers FP, Schwab ME. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Gahtan E, Ubeda O, Beech W, Overmier JB, Hsiao-Ashec K, Frautschy SA, Cole GM. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol Aging. 2001;22:983–991. doi: 10.1016/s0197-4580(01)00299-8. [DOI] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelinassociated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Liu BP, Cafferty WB, Budel SO, Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond Biol. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord-Fontaine S, Yang F, Diep Q, Dergham P, Munzer S, Tremblay P, McKerracher L. Local inhibition of Rho signaling by cellpermeable recombinant protein BA-210 prevents secondary damage and promotes functional recovery following acute spinal cord injury. J Neurotrauma. 2008;25:1309–1322. doi: 10.1089/neu.2008.0613. [DOI] [PubMed] [Google Scholar]
- Mandula H, Parepally JM, Feng R, Smith QR. Role of site-specific binding to plasma albumin in drug availability to brain. J Pharmacol Exp Ther. 2006;317:667–675. doi: 10.1124/jpet.105.097402. [DOI] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- McKerracher L, Higuchi H. Targeting Rho to stimulate repair after spinal cord injury. J Neurotrauma. 2006;23:309–317. doi: 10.1089/neu.2006.23.309. [DOI] [PubMed] [Google Scholar]
- McTigue DM. Potential therapeutic targets for PPARgamma after spinal cord injury. PPAR Res. 2008;2008:517162. doi: 10.1155/2008/517162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz GA, Curt A, Meent H van de, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000a;17:1–17. doi: 10.1089/neu.2000.17.1. [DOI] [PubMed] [Google Scholar]
- Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000b;883:165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao ZH, Thill G, Ji BX, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate RL, McCoy JM, Pepinsky RB. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RF, Adams SS, Cliffe EE, Dickinson W, Nicholson JS. The metabolism of ibuprofen. Xenobiotica. 1973;3:589–598. doi: 10.3109/00498257309151547. [DOI] [PubMed] [Google Scholar]
- Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res. 2002;137:313–332. doi: 10.1016/s0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- Muellner A, Gonzenbach RR, Weinmann O, Schnell L, Liebscher T, Schwab ME. Lamina-specific restoration of serotonergic projections after Nogo-A antibody treatment of spinal cord injury in rats. Eur J Neurosci. 2008;27:326–333. doi: 10.1111/j.1460-9568.2007.06006.x. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Fujita Y, Ueno M, Takai T, Yamashita T. Paired immunoglobulin-like receptor B knockout does not enhance axonal regeneration or locomotor recovery after spinal cord injury. J Biol Chem. 2011;286:1876–1883. doi: 10.1074/jbc.M110.163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa M, Aasato M, Hayashi SS, Kamei J. RhoA/Rho kinase pathway contributes to the pathogenesis of thermal hyperalgesia in diabetic mice. Pain. 2011;152:114–122. doi: 10.1016/j.pain.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Omoto S, Ueno M, Mochio S, Takai T, Yamashita T. Genetic deletion of paired immunoglobulin-like receptor B does not promote axonal plasticity or functional recovery after traumatic brain injury. J Neurosci. 2010;30:13045–13052. doi: 10.1523/JNEUROSCI.3228-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka S, Adamson C, Sankar V, Gibbs KM, Kane-Goldsmith N, Ayer J, Babiarz J, Kalinski H, Ashush H, Alpert E, Lahav R, Feinstein E, Grumet M. Delayed intrathecal delivery of RhoA siRNA to the contused spinal cord inhibits allodynia, preserves white matter, and increases serotonergic fiber growth. J Neurotrauma. 2011;28:1063–1076. doi: 10.1089/neu.2010.1568. [DOI] [PubMed] [Google Scholar]
- Pantovic R, Draganic P, ErakovicV BB, Milin C, Simonic A. Effect of indomethacin on motor activity and spinal cord free fatty acid content after experimental spinal cord injury in rabbits. Spinal Cord. 2005;43:519–526. doi: 10.1038/sj.sc.3101763. [DOI] [PubMed] [Google Scholar]
- Parepally JM, Mandula H, Smith QR. Brain uptake of nonsteroidal anti-inflammatory drugs: ibuprofen, flurbiprofen, and indomethacin. Pharm Res. 2006;23:873–881. doi: 10.1007/s11095-006-9905-5. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Deitinghoff L, Davis D, Conrad S, Skutella T, Chedotal A, Mueller BK, Strittmatter SM. Neogenin mediates the action of repulsive guidance molecule. Nat Cell Biol. 2004;6:756–762. doi: 10.1038/ncb1156. [DOI] [PubMed] [Google Scholar]
- Ramer LM, Borisoff JF, Ramer MS. Rho-kinase inhibition enhances axonal plasticity and attenuates cold hyperalgesia after dorsal rhizotomy. J Neurosci. 2004;24:10796–10805. doi: 10.1523/JNEUROSCI.3337-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robak LA, Venkatesh K, Lee H, Raiker SJ, Duan Y, Lee-Osbourne J, Hofer T, Mage RG, Rader C, Giger RJ. Molecular basis of the interactions of the Nogo-66 receptor and its homolog NgR2 with myelin-associated glycoprotein: development of NgROMNI-Fc, a novel antagonist of CNS myelin inhibition. J Neurosci. 2009;29:5768–5783. doi: 10.1523/JNEUROSCI.4935-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Schwab M, Schwartz M, Fehlings MG. Spinal cord injury: time to move? J Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Ikegaki I, Suzuki Y, Asano T, Shibuya M, Hidaka H. Neuroprotective properties of a protein kinase inhibitor against ischaemia-induced neuronal damage in rats and gerbils. Br J Pharmacol. 1996;118:1592–1596. doi: 10.1111/j.1476-5381.1996.tb15579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Toshima Y, Hitomi A, Ikegaki I, Seto M, Asano T. Wide therapeutic time window for Rho-kinase inhibition therapy in ischemic brain damage in a rat cerebral thrombosis model. Brain Res. 2008;1193:102–108. doi: 10.1016/j.brainres.2007.11.050. [DOI] [PubMed] [Google Scholar]
- Schafer AI. Effects of nonsteroidal anti-inflammatory therapy on platelets. Am J Med. 1999;106:25S–36S. doi: 10.1016/s0002-9343(99)00114-x. [DOI] [PubMed] [Google Scholar]
- Scheuren N, Bang H, Münster T, Brune K, Pahl A. Modulation of transcription factor NF-kappaB by enantiomers of the nonsteroidal drug ibuprofen. Br J Pharmacol. 1998;123:645–652. doi: 10.1038/sj.bjp.0701652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmandke A, Schmandke A, Strittmatter SM. ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist. 2007;13:454–469. doi: 10.1177/1073858407303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Hirsch S, Monnier PP, Brechtel K, Stiefel A, Leppert CA, Schluesener HJ, Barth H, Aktories K, Mueller BK. Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2002. The Rho-GTPase inhibitor C3-C2IN/C2II induces functional neuronal recovery in a rat model of severe spinal cord injury. Program No. 204.7. [Google Scholar]
- Schwab JM, Conrad S, Elbert T, Trautmann K, Meyermann R, Schluesener HJ. Lesional RhoA + cell numbers are suppressed by anti-inflammatory, cyclooxygenase-inhibiting treatment following subacute spinal cord injury. Glia. 2004;47:377–386. doi: 10.1002/glia.20031. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Guo L, Schluesener HJ. Spinal cord injury induces early and persistent lesional P2X4 receptor expression. J Neuroimmunol. 2005;163:185–189. doi: 10.1016/j.jneuroim.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Tuli SK, Failli V. The Nogo receptor complex: confining molecules to molecular mechanisms. Trends Mol Med. 2006;12:293–297. doi: 10.1016/j.molmed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Schweigreiter R, Bandtlow CE. Nogo in the injured spinal cord. J Neurotrauma. 2006;23:384–396. doi: 10.1089/neu.2006.23.384. [DOI] [PubMed] [Google Scholar]
- Seymour AB, Andrews EM, Tsai SY, Markus TM, Bollnow MR, Brenneman MM, O'Brien TE, Castro AJ, Schwab ME, Kartje GL. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, Murray B, Jung V, Pepinsky RB, Mi S. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Winkler T. Assessment of spinal cord pathology following trauma using early changes in the spinal cord evoked potentials: a pharmacological and morphological study in the rat. Muscle Nerve. 2002;25(Suppl 11):S83–S91. doi: 10.1002/mus.10152. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Olsson Y, Cervos-Navarro J. Early perifocal cell changes and edema in traumatic injury of the spinal cord are reduced by indomethacin, an inhibitor of prostaglandin synthesis. Experimental study in the rat. Acta Neuropathol. 1993a;85:145–153. doi: 10.1007/BF00227761. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Olsson Y, Nyberg F, Dey PK. Prostaglandins modulate alterations of microvascular permeability, blood flow, edema and serotonin levels following spinal cord injury: an experimental study in the rat. Neuroscience. 1993b;57:443–449. doi: 10.1016/0306-4522(93)90076-r. [DOI] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E, Fasudil Ischemic Stroke Study Group Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005;238:31–39. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Shin HK, Salomone S, Potts EM, Lee SW, Millican E, Noma K, Huang PL, Boas DA, Liao JK, Moskowitz MA, Ayata C. Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow Metab. 2007;27:998–1009. doi: 10.1038/sj.jcbfm.9600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Taylor DA, McClelland JM, Rutkowski SB, Cousins MJ. Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain. 1999;81:187–197. doi: 10.1016/s0304-3959(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Simpson RK, Jr, Baskin DS, Dudley AW, Bogue L, Rothenberg F. The influence of long-term nifedipine or indomethacin therapy on neurologic recovery from experimental spinal cord injury. J Spinal Disord. 1991;4:420–427. doi: 10.1097/00002517-199112000-00003. [DOI] [PubMed] [Google Scholar]
- Sing G, Ramey DR. NSAID induced gastrointestinal complications: The ARAMIS Perspective-1997. J Rheumatol. 1998;25(Suppl 51):8–16. [PubMed] [Google Scholar]
- Tanaka H, Yamashita T, Yachi K, Fujiwara T, Yoshikawa H, Tohyama M. Cytoplasmic p21(Cip1/WAF1) enhances axonal regeneration and functional recovery after spinal cord injury in rats. Neuroscience. 2004;127:155–164. doi: 10.1016/j.neuroscience.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. [DOI] [PubMed] [Google Scholar]
- Tatsumi S, Mabuchi T, Katano T, Matsumura S, Abe T, Hidaka H, Suzuki M, Sasaki Y, Minami T, Ito S. Involvement of Rho-kinase in inflammatory and neuropathic pain through phosphorylation of myristoylated alanine-rich C-kinase substrate (MARCKS) Neuroscience. 2005;131:491–498. doi: 10.1016/j.neuroscience.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Thoenen H, Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat Neurosci. 2002;5(Suppl):1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- Toshima Y, Satoh S, Ikegaki I, Asano T. A new model of cerebral microthrombosis in rats and the neuroprotective effect of a Rho-kinase inhibitor. Stroke. 2000;31:2245–2250. doi: 10.1161/01.str.31.9.2245. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Toyomitsu E, Komatsu T, Masuda T, Kunifusa E, Nasu-Tada K, Koizumi S, Yamamoto K, Ando J, Inoue K. Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia. 2008;56:579–585. doi: 10.1002/glia.20641. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wang X, Baughman KW, Basso DM, Strittmatter SM. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol. 2006;60:540–549. doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Budel S, Baughman GG, Song KH, Strittmatter SM. Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. J Neurotrauma. 2009;26:81–95. doi: 10.1089/neu.2007.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Golde TE, Koo EH. Abeta42-lowering nonsteroidal anti-inflammatory drugs preserve intramembrane cleavage of the amyloid precursor protein (APP) and ErbB-4 receptor and signaling through the APP intracellular domain. J Biol Chem. 2003;278:30748–30754. doi: 10.1074/jbc.M304824200. [DOI] [PubMed] [Google Scholar]
- Whelton A. Nephrotoxicity of nonstreoidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106:13S–24S. doi: 10.1016/s0002-9343(99)00113-8. [DOI] [PubMed] [Google Scholar]
- Winkler T, Sharma HS, Stalberg E, Olsson Y. Indomethacin, an inhibitor of prostaglandin synthesis attenuates alteration in spinal cord evoked potentials and edema formation after trauma to the spinal cord: an experimental study in the rat. Neuroscience. 1993;52:1057–1067. doi: 10.1016/0306-4522(93)90552-q. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Pettegrew RK, Harvey F. Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp Neurol. 1985;88:108–122. doi: 10.1016/0014-4886(85)90117-7. [DOI] [PubMed] [Google Scholar]
- Wu D, Yang P, Zhang X, Luo J, Haque ME, Yeh J, Richardson PM, Zhang Y, Bo X. Targeting a dominant negative rho kinase to neurons promotes axonal outgrowth and partial functional recovery after rat rubrospinal tract lesion. Mol Ther. 2009;17:2020–2030. doi: 10.1038/mt.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Zheng B. White matter inhibitors in CNS axon regeneration failure. Exp Neurol. 2008;209:302–312. doi: 10.1016/j.expneurol.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Li H, Wang H, Mukhopadhyay D, Fisher D, Gilpin CJ, Li S. RhoA-inhibiting NSAIDs promote axonal myelination after spinal cord injury. Exp Neurol. 2011;231:247–260. doi: 10.1016/j.expneurol.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Fujitani M, Hata K, Kitajo K, Mimura F, Abe H, Yamashita T. Wallerian degeneration involves Rho/Rho-kinase signaling. J Biol Chem. 2005;280:20384–20388. doi: 10.1074/jbc.M501945200. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Kotani Y, Nakajima Y, Shimazawa M, Yoshimura S, Nakashima S, Iwama T, Hara H. Fasudil, a Rho kinase (ROCK) inhibitor, protects against ischemic neuronal damage in vitro and in vivo by acting directly on neurons. Brain Res. 2007;1154:215–224. doi: 10.1016/j.brainres.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, Paul SM, Ni B. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci. 2011;32:167–173. doi: 10.1016/j.tips.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]