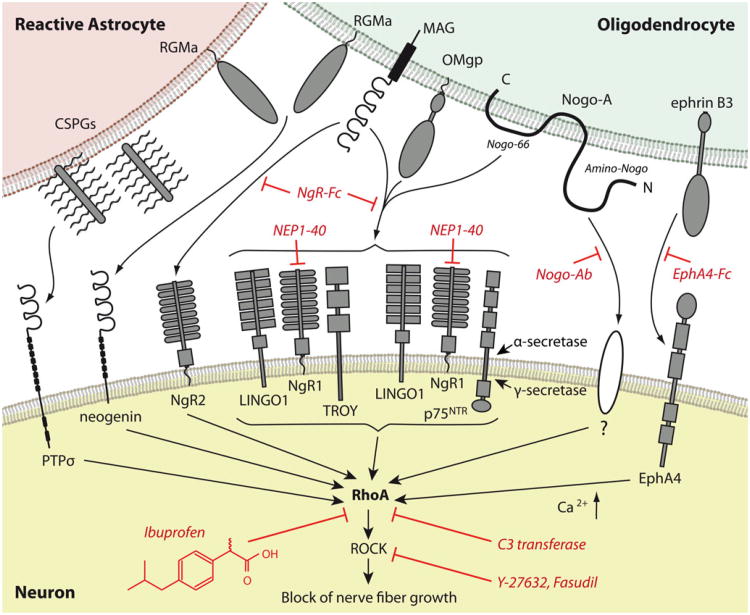
Fig. 1.
Inhibitory molecules and therapeutic targets within the Rhopathway. Diverse growth inhibitory molecules from myelin and reactive astrocytes interact with various receptors signaling through a converging downstream pathway within the axon after injury to the central nervous system. The activation of RhoA represents a key mechanism of this cascade. NgR1 forms a receptor complex with LINGO1 and p75NTR or TROY, which is required for Nogo-66 signaling. Cleavage of p75NTR through α-secretase and γ-secretase leads to the release of its intracellular domain, which transmits the inhibitory signal. The Amino-Nogo sequence of Nogo-A activates RhoA through an unknown receptor. The increase in intracellular calcium is involved in RhoA-activation. Interference with RhoA-activation blocks the integration of the inhibitory signal into the injured axon and results in axonal re-growth. At the upstream level, Nogo antibodies prevent the inhibition of Nogo-A-mediated outgrowth. Soluble NgR-Fc or NgROMNI -Fc block the binding of myelin inhibitors to NgR1 or NgR2 and the NgR1 agonist NEP1-40 also interferes with binding to NgR1. Ephrin signaling can be blocked by unclustered EphA4-Fc. RhoA-activation itself is inhibited by C3 transferase and by “small molecules”, such as the NSAID ibuprofen. Y27623 and fasudil inhibit the downstream target ROCK (CSPGs chondroitin sulfate proteoglycans, MAG myelin-associated glycoprotein, OMgp oligodendrocyte-myelin glycoprotein, ROCK Rho-kinase, RGMa repulsive guidance molecule–A)