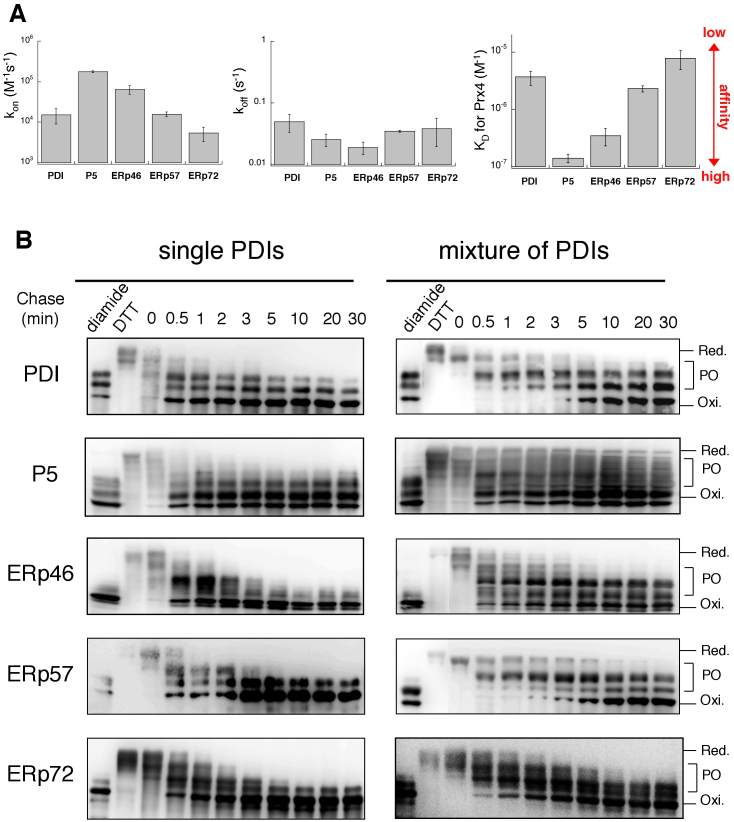
Figure 2. Preferential oxidation of ERp46 and P5 by Prx4 in vitro.
(A) SPR analysis of the affinity of PDIs for Prx4. The bar graphs show the association (kon) and dissociation (koff) rate constants and KD values of PDIs for Prx4 (see also Supplementary Fig. S4). Values are the mean ± SD of three independent experiments. (B) Reduced form(s) of a single PDI family member or a subset of PDIs (10 μM each) were reacted with Prx4 (0.2 μM) in buffer (pH 7.5) containing 300 μM H2O2. The reaction was quenched by cysteine alkylation with malPEG2K, and the generated species were resolved by non-reducing SDS-PAGE and visualized by immunoblotting with the indicated antibodies. The representative gel images of the three independent experiments are shown. Labels Red., PO, and Oxi. denote fully reduced, partially oxidized, and fully oxidized forms of PDIs, respectively. The blotting data are cropped to highlight the time course of Prx4-catalyzed oxidation of each PDIs.