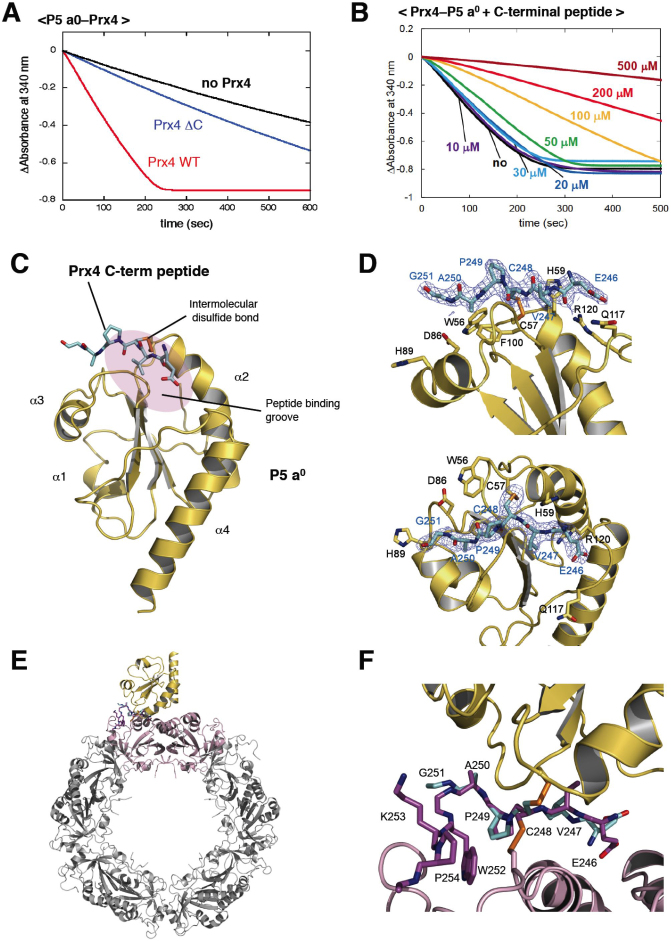
Figure 3. Mode of interaction between Prx4 and Trx of P5.
(A) Activity of the C-terminally truncated Prx4 mutant (Prx4 ΔC) toward P5 a0. The experimental conditions were as described in Materials and Methods. Consumption of NADPH coupled to Prx4 catalysis was detected by monitoring absorbance at 340 nm (Abs340). (B) Inhibition of Prx4-catalyzed oxidation of P5 a0 by the titration of the Prx4 C-terminal peptide. The experiments were carried out as in (a) in the presence of various concentrations (0, 10, 20, 30, 50, 100, 200, 300, 500 μM) of the Prx4 C-terminal peptide. (C) Crystal structure of P5 a0 (yellow) in a complex with the Prx4 C-terminal peptide (cyan) at 2.1 Å resolution. The Prx4 peptide is shown with sticks. Cys57 in the redox-active site of P5 a0 formed a mixed disulfide bond with the resolving cysteine (Cys248) of Prx4, as illustrated by orange sticks. The peptide-binding groove of P5 a0 is shown with a purple oval. (D) Close-up views of the interface of the P5 a0–Prx4 C-terminal peptide complex from two different angles. Electron density map at the 1.0 contour level of the Prx4 C-terminal peptide is indicated with blue mesh. (E) Modeled structure of the P5 a0–Prx4 decamer complex. The Prx4 C-terminal peptide bound to P5 a0 is superposed on the corresponding part of the oxidized Prx4 decamer such that the RMSD of the Cα atoms of Val247, Cys248, Pro249, and Ala250 is minimized. A dimeric unit of Prx4 that interacts with P5 a0 is highlighted in pink. (F) Close-up view of the interface of the P5 a0–Prx4 decamer complex shown in (E).