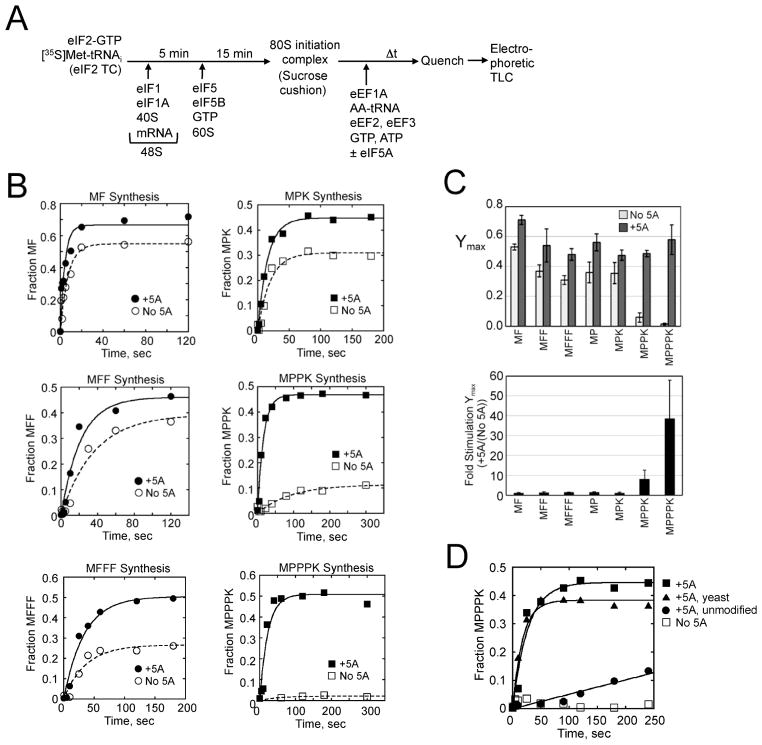
Figure 4. eIF5A Stimulates Synthesis of Polyproline Peptides.
(A) Scheme for in vitro reconstituted translation elongation assay.
(B) Fractions of MF, MFF, MFFF (left column) or MPK, MPPK, and MPPPK (right) synthesis in elongation assays (Fig. S3D–E) performed in the absence (open symbols) or presence of eIF5A (closed symbols) were plotted and fit to a single exponential equation.
(C) Summary of maximum fractions of peptide synthesis (Ymax, top) and fold stimulation of Ymax by adding eIF5A (bottom) calculated from the data in panel B. Error bars are (upper) SD from at least three independent experiments and (lower) calculated propagated SD.
(D) Effect of eIF5A hypusine modification on peptide synthesis. Fraction of MPPPK synthesis (Fig. S3F) in reactions lacking eIF5A, containing unmodified eIF5A (no hypusine), or containing hypusinated eIF5A prepared from E. coli (+5A, see Experimental Procedures) or purified from yeast (+5A, yeast) was plotted and fit to a single exponential equation.