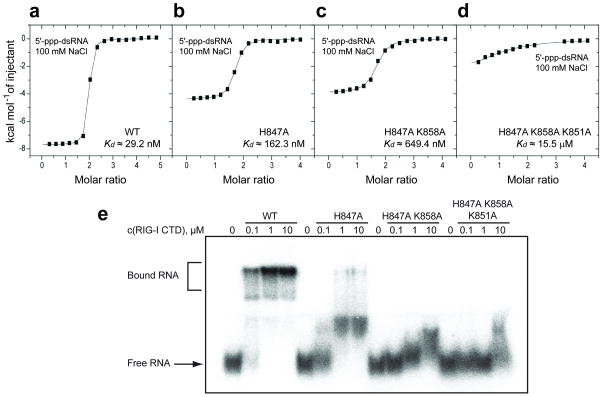
Figure 4.
ITC and electrophoretic mobility shift studies of the binding of blunt-end 5’-ppp-dsRNA 12-mer to wild-type and mutants of RIG-I CTD. ITC binding curves for blunt-end 5’-ppp-dsRNA 12-mer binding to (a) wild-type, (b) H847A single mutant, (c) H847A/K858A double mutant and (d) H847A/K858A/K851A triple mutant of RIG-I CTD domain in 100 mM NaCl and 2 mM MgCl2 buffer. (e) Palindromic radiolabeled 5’-ppp-dsRNA 12-mer was incubated at increasing concentrations of recombinant protein under the same salt and buffer conditions as the ITC experiment. The complexes were resolved on a native polyacrylamide gel. Increasing numbers of mutation in the triphosphate-binding pocket of the protein weaken RNA binding and reduce the distinct gel shift seen for the wild-type protein to a smear. To ensure that the palindromic 5’-ppp-RNA was exclusively present as dsRNA rather than a hairpin, we pre-annealed the RNA at 5 μM strand concentration and only diluted it to 20 nM prior to the incubation with protein.