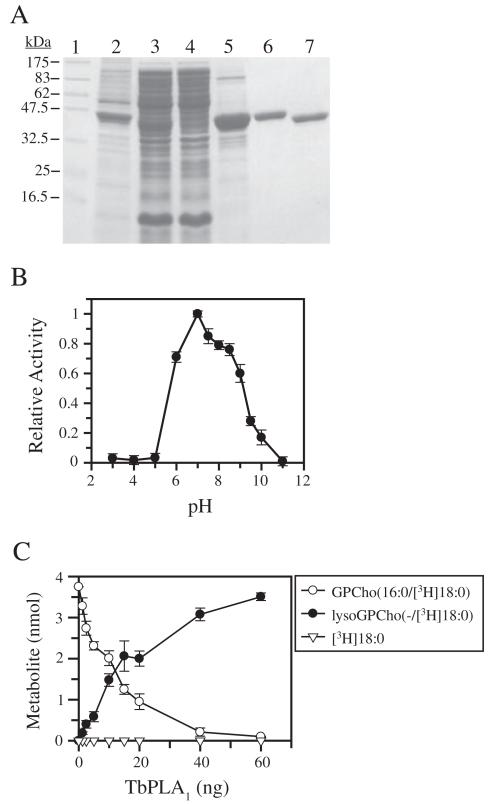
Fig. 2.
Purification and regiospecificity of recombinant TbPLA1.
A. SDS-PAGE analysis of proteins from TbPLA1-overexpressing E. coli cells during various purification steps as compared with molecular weight standards (lane 1). The various fractions include insoluble proteins (lane 2), the soluble protein fraction containing His·tagged recombinant TbPLA1 (lane 3), nickel column flow through (lane 4), nickel affinity eluate (lane 5), purified His·tagged recombinant TbPLA1 after anion exchange (~3 μg) (lane 6), purified His·tag-cleaved TbPLA1 after thrombin digestion and anion exchange (~3 μg) (lane 7).
B. Phospholipase activity was measured at various pH values using the fluorescent BODIPY®C11-PC assay at a mole fraction of 0.018 with a final concentration of substrate at 0.075 mM. A universal buffer containing 50 mM Tri-sodium citrate, 50 mM Tris and 50 mM NaCl was used to obtain the various pH conditions. Data points represent the activity measured relative to the maximal pH-activity value of pH 7.0. Data represent the average from two experiments performed in triplicate.
C. Metabolism of GPCho(16:0/[3H]18:0) after a 5 min reaction is shown as a function of varying amounts of recombinant TbPLA1. Formation of lysoGPCho(−/[3H]18:0) appears to be dose-dependent, whereas [3H]18:0 acid cleavage from the sn-2 position is undetectable.