Abstract
Objective:
Adjuvant radiation therapy (RT) is an essential part of combined limb-sparing treatment of soft-tissue sarcoma (STS). Elderly or medically unfit patients often have difficulty in completing 6–7 weeks of standard fractionated daily treatment. Our aim was to evaluate the efficacy of a hypofractionated adjuvant approach with RT for STS in elderly and debilitated patients.
Methods:
21 elderly patients were treated with a short course of adjuvant RT (39–48 Gy, 3 Gy per fraction) for STS. The medical records of the patients were retrospectively reviewed for local or distant recurrence and side effects of RT.
Results:
At a mean 26 months of follow-up, three local recurrences (14%) were detected. Eight patients (38%) had lung metastases during the observed period. Three of them died from metastatic disease. The hypofractionated radiation was well tolerated with minimum long-term side effects.
Conclusion:
Hypofractionated adjuvant radiation appears to be an effective treatment in terms of local control in elderly and debilitated patients.
Advances in knowledge:
The results of this study might provide an alternative to commonly used standard fractionation of radiotherapy in sarcoma patients.
Soft-tissue sarcoma (STS) is a relatively rare disease. The age-adjusted incidence rate is 3.3 per 100 000 males and females per year. From 2005 to 2009, the median age at diagnosis for cancers of the soft tissue was 58 years. Approximately 16.1% were diagnosed between 65 and 74, 16.3% between 75 and 84 and 7.3% at 85+ years old [1].
The modern approach to high-grade limb STS in adults is based on limb-sparing surgery followed by radiation therapy (RT). The benefit of RT in terms of local control was documented in two randomised trials [2,3]. The indications for pre-operative or post-operative chemotherapy are not sufficiently clear at this juncture. Adjuvant RT is an essential part of combined limb-sparing treatment of STS. The recommended dose of radiation lies in the range of 60 Gy in standard fractionation of 1.8–2.0 Gy. Elderly or medically unfit patients often have difficulty in completing 6–7 weeks of daily treatment. Moreover, a prolonged course of radiation may be interrupted by acute side effects, which sometimes demands further extension of the overall course or even discontinuation of treatment.
We retrospectively studied the rate of local control and distant metastases in elderly patients with STS treated by short-course adjuvant radiation.
PATIENTS AND METHODS
The patients’ characteristics are presented in Table 1. 21 elderly or medically unfit patients diagnosed with STS were treated by RT following curative surgery. There were 13 males and 8 females. The median age was 80 years. The most common comorbidities were hypertension, diabetes mellitus and cognitive disorders (Table 1). The decision to treat patients with a hypofractionated regimen was based on the clinical assessment by physicians. Inclusion criteria included functional performance status >0 (Eastern Cooperative Oncology Group scale), large distance from the medical centre or a patient’s desire to receive the short course of treatment as an alternative to the conventional/longer course that they refused to accept. The types of sarcoma included malignant fibrohistiocytoma (two), liposarcoma (two), leiomyosarcoma (three), pleomorphic sarcoma (seven), fibrosarcoma (six) and synovial sarcoma (one). The anatomical distribution of the primary tumours were thigh (seven), calf (five), arm (four), shoulder (two) and pelvis (one). Most of the patients underwent marginal excision of tumour (17). No widening of surgical margins was attempted owing to the general condition of the patients. In 15 patients, the surgical margins were <5 mm, 4 of them were involved by microscopic disease.
Table 1.
Patients’ demographics and tumour characteristics
| No. | Gender | Age (years) | PS | Comorbidity | Site | Type STS | Size (cm) | Type of surgery | Margins | Days before start of radiation therapy |
| 1 | M | 90 | 1 | HTN, BPH, LHR | L calf | MFH | 3.5 | WLE | 0.4 cm | 108 |
| 2 | F | 82 | 1 | HTN, BPH, LHR | R thigh | WDLS | 10.0 | Marginal (sk graft) | Positive | 69 |
| 3 | F | 68 | 1 | Cardiac myxoma | R thigh | Rec LMS | 8.0 | Marginal | 1.0 mm | 74 |
| 4 | M | 76 | 1 | HTN, CRF, BPH | L arm | Pleom HG | 19.0 | Marginal | Positive | 42 |
| 5 | M | 80 | 1 | COPD | L arm | MFH | 16.0 | Marginal | 2.0 mm | 48 |
| 6 | M | 86 | 0 | IHD, NIDDM | L arm | MyxoFS | 5.0 | WLE | 2.5 cm | 83 |
| 7 | M | 75 | 2 | HTN, IHD | L pelv | MyxoFS | 20.0 | Marginal | 2.0 mm | 90 |
| 8 | F | 76 | 1 | HTN, NIDDM, Alzheimer | L thigh | LS | 4.5 | Marginal | 4.0 mm | 70 |
| 9 | M | 87 | 2 | Gout | R shoulder | Rec Pleom HG | 2.3 | Marginal | 2.0 mm | 48 |
| 10 | M | 85 | 2 | HTN, CVA | L calf | LMS | 8.0 | Marginal (sk graft) | 3.0 mm | 67 |
| 11 | F | 89 | 2 | COPD, Ca of uterus | R knee | Rec FS | 8.0 | Marginal (sk graft) | 3.0 mm | 50 |
| 12 | M | 87 | 1 | NHL, IHD | R thigh | Pleom HG | 19.0 | Marginal | 1.0 mm | 42 |
| 13 | M | 66 | 3 | L arm | LMS | 1.5 | WLE | 1.0 cm | 90 | |
| 14 | M | 86 | 2 | HTN, BPH, LHR | L thigh | FS | 30.0 | WLE | 1.0 cm | 61 |
| 15 | M | 56 | 1 | HIV, low compliance | L shoulder | SS | 14.0 | Marginal | Positive | 57 |
| 16 | M | 80 | 1 | HTN, BPH, AF, Parkinson | L calf | Pleom HG | 6.0 | Marginal | ? | 20 |
| 17 | F | 78 | 2 | Bipolar ds, Ca of breast | L thigh | Pleom HG | 7.0×5.0×2.0 | Marginal | 1.0 mm | 62 |
| 18 | F | 89 | 1 | HTN, IHD, COPD, goitre | R forearm | FS | 8.0×5.0×5.0 | Marginal | 1.0 mm | 74 |
| 19 | M | 86 | 1 | IHD, CRF, CHF, Pulm HTN | R thigh | Pleom HG | 10.0×5.0×4.0 | Marginal | 2.0 mm | 63 |
| 20 | F | 76 | 1 | HTN, DM, CRF | R calf | MyxoFS | 2.5 cm | Marginal | 5.0 mm | 46 |
| 21 | F | 80 | 2 | Peptic ds, OA | L calf | Pleom HG | 12.0×7.0×6.0 | Marginal | Positive | 102 |
AF, atrial fibrillation; BPH, benign prostatic hyperplasia; Ca, cancer; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; CVA, cerebral vascular accident; DM, diabetes mellitus; ds, disorder; F, female; FS, fibrosarcoma; HG, high grade; HIV, human immunodeficiency virus; HTN, hypertension; IHD, ischemic heart disease; L, left; LHR, left hip replacement; LMS, leiomyosarcoma; LS, liposarcoma; M, male; MFH, malignant fibrous histiocytoma, MyxoFS, mixoid fibrosarcoma; NHL, non-Hodgkin lymphoma; NIDDM, non-insulin dependant diabetes mellitus; No., number; OA, osteoarthritis; Pulm, pulmonary; PS, performance status; R, right; sk, skin; SS, synovial sarcoma; STS, soft-tissue sarcoma; WDLS, well-differentiated liposarcoma; WLE, wide local excision.
Most tumours had a high-grade malignancy (Grades 3–17) and were >5 cm in size (16), with 8 of them >10 cm.
All patients underwent CT simulation (Brilliance Big Bore; Philips, Best, Netherlands). The positioning and immobilisation of patients was individualised as a function of tumour location. Beams were designed to achieve coverage of the clinical treatment volume (CTV) based on the pre-operative CT scan or MRI and clips that surgeons placed at the tumour bed and its edges. Traditionally, the borders of the CTV were arranged in extension of 2–5 cm beyond the pre-operative tumour volume depending on the margin status. Treatment planning was carried out with the XiO® system (CMS Corporation, St. Louis, MO) and was consonant with requirements of the International Commission on Radiation Units & Measurements report 50 [4]. Before the start of treatment, each patient had verification of portals (I-View™ GT-IVIEW02; Eleka, Crawley, UK). The radiation was administered with Elekta (Stockholm, Sweden) linear accelerators with energies of 6 MV or 18 MV. Patients were assessed for side effects and compliance with treatment on a weekly basis during therapy: at the end of treatment, and every 3 months thereafter. All patients were treated by a short and intensive course of therapy: 39 Gy was given in 13 fractions of 3 Gy day−1, 5 times a week to patients with closest surgical margins of at least 5 mm, whereas a dose of 48 Gy given in 16 fractions was provided in cases of margins that were closer than 5 mm.
The patients were scheduled for follow-up according to European Society for Medical Oncology guidelines [5] for regular physical examination every 3–4 months after completion of RT in addition to semi-annual MRI or CT scans of the treated site and CT scanning of the chest. The date of local recurrence or first distant metastases was registered for calculation of the disease-free progression and the rate of local control.
RESULTS
Overall, the hypofractionated irradiation regimen of 39–48 Gy in 13–16 fractions was well tolerated with only 3 patients developing Grade 2 or 3 acute toxicity (mainly dermatitis). Three patients had delayed Grade 2 or 3 toxicity (chronic pain, skin atrophy, telangiectasia) scaled according to common toxicity criteria [6].
The mean time from surgery until the initiation of RT was 2.1 months [standard deviation (SD) 0.7]. Mean radiation treatment time was 18.4 days (SD 3). No delay in treatment owing to acute toxicity was registered. All patients except for one were able to receive RT in the ambulatory setting.
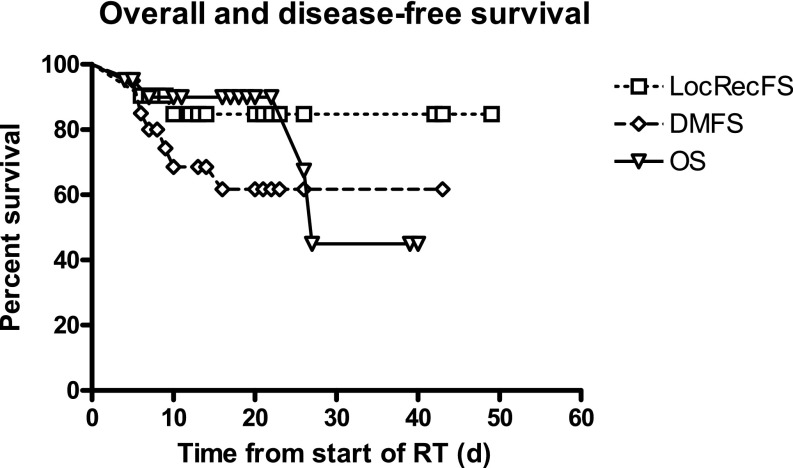
With a mean follow-up of 26 months (SD 10.7), three local recurrences (14%) were detected (all in patients with surgical margins <3 mm). Three of eight patients with distant metastases died of sarcoma (Figure 1). One patient with metastatic disease in the lung received salvage stereotactic RT and was still alive 6 months after completion of stereotactic body radiotherapy with no evidence of disease. The Kaplan Meier curves for overall and disease-free survival were plotted, and the results were similar to those of soft-tissue sarcoma patients treated with standard fractionation (Figure 1).
Figure 1.
Overall and disease-free survival of patients with soft-tissue sarcoma treated by hypofractionated radiation therapy. DMFS, distant metastases free survival; LocRecFS, local regional free survival; OS, overall survival; RT, radiotherapy.
DISCUSSION
Our data point to the feasibility, safety and efficacy of adjuvant hypofractionated RT following limb-sparing surgery in adults with high-grade STS of the limb. The local control rate was 86%, which is similar to results of usually recommended adjuvant treatment in standard 1.8–2.0-Gy fractionation [7].
The hypofractionated treatment seems to be especially suitable for elderly patients with significant comorbidities. None of the patients interrupted treatment owing to underlying diseases or poor compliance.
In view of their age and underlying medical condition, the patients in the current study represent a cohort with undesirable risk factors in relation to expected compliance and tolerability. Most of them refused the proposed standard 6–7-week course of adjuvant RT owing to complicated logistics or comorbidities. Our results show good tolerability and acceptable 2-year local control with the short course of adjuvant RT among debilitated or elderly patients with STS.
A shorter course of RT is much easier for patients and for accompanying family members or caregivers. In the presence of acceptable efficacy and tolerability, a shorter course of RT may also be economically justified.
In a recent report from the Memorial Sloan–Kettering Cancer Center, the 5-year local recurrence rate was 9%. Old age and stage 3 disease were identified as factors associated with a higher rate of local recurrence [8].
From a radiobiological perspective, sarcomas are usually considered as poorly to moderately radioresponsive tumours. RT doses in the range of 60–70 Gy are usually necessary in order to eradicate microscopic disease. One of the biological characteristics of sarcoma cells is their relatively low (−0.5 to 5.4) α–β ratio [9]. This ratio, theoretically, may justify the use of larger-than-standard fractionation in order to achieve significant cell kill by RT.
Assuming an α–β ratio of sarcoma cells of 4, our calculation of the equivalent dose in 2-Gy fractions (EQD2) resulted in 46 Gy and 56 Gy, respectively, for 13 and 16 fractions of 3 Gy using the formula of EQD2=D[d+α/β]/[2+α/β] [9]. In cases of margins closer than 5 mm or frankly positive margins, we augmented the dose to 48 Gy in 16 fractions. Moreover, the use of hypofractionation in adjuvant RT is becoming widely adopted in the treatment of different solid tumours [10,11]. The historical assumption that standard fractionation of 1.8–2.0 Gy is acceptable for most malignant tumours is therefore being re-assessed by many clinical investigators.
In our previous publication [12], we reported good tolerance and high efficacy of the same hypofractionated regimen among patients with metastatic STS in terms of local control for macroscopic disease.
Eilber et al [13] first applied a hypofractionation regimen for treatment of STS.
Ryan et al [14] achieved a high rate of pathological tumour necrosis (95%) following 28 Gy administered in eight fractions. We found these regimens to be intriguing and worthy of further study.
We previously reported good tolerance and results with a short course of palliative radiation delivered to unfit patients with STSs. This prompted us to consider applying a similar regimen in the adjuvant setting. This new option is worthy of consideration for a challenging population (i.e. elderly/medically unfit) for whom some would contemplate foregoing irradiation despite the consensus that RT is a critical therapeutic component [3,7]. Omitting RT in a patient treated with limb-sparing surgery increases the risk of local recurrence [7]. As an alternative to limb-sparing surgery without RT, amputation surgery may be suggested, hampering the quality of life even more, and possibly rendering the patient bed-ridden. A short and intensive course of RT may be a logical and adequate solution to this dilemma.
An interesting dimension of our study was the ability to achieve high rates of local control despite a surgical policy that did not insist on attaining negative margins. This is particularly noteworthy in light of the emphasis on the importance of margin status by McKee et al [15]. At the same time, it is intriguing that the previous emphasis on the importance of margin status is being revisited throughout oncology [16]. Whether the use of hypofractionated regimes such as the one employed at our institution can overcome the significance of involved or close margins is worthy of further study.
The limitations of the current study are the small number of patients and the relatively short observation time. Since our conclusions are not attributable to a database of prospectively randomised patients, our findings are only hypothesis generating. Notwithstanding, the promising results might provide insight into revision of the standard fractionation used in the field of soft-tissue malignancy.
REFERENCES
- 1.National Cancer Institute. SEER stat fact sheets: soft tissue including heart. Incidence & Mortality. Available from: http://seer.cancer.gov/statfacts/html/soft.html#incidence-mortality.
- 2.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859. [DOI] [PubMed] [Google Scholar]
- 3.Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197. [DOI] [PubMed] [Google Scholar]
- 4.International Commission on Radiation Units & Measurements Prescribing, recording, and reporting photon beam therapy. Report 50. Bethesda, MD: ICRU; 1993 [Google Scholar]
- 5.Casali PG, Jost L, Sleijfer S, Verweij J, Blay JY. Soft tissue sarcomas: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20:iv132–6 [DOI] [PubMed] [Google Scholar]
- 6.Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, et al. Common toxicity criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 2000;47:13–47 [DOI] [PubMed] [Google Scholar]
- 7.Strander H, Turesson I, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol 2003;42:516–31 [DOI] [PubMed] [Google Scholar]
- 8.Cahlon O, Spierer M, Brennan MF, Singer S, Alektiar KM. Long-term outcomes in extremity soft tissue sarcoma after a pathologically negative re-resection and without radiotherapy. Cancer 2008;112:2774–9 10.1002/cncr.23493 [DOI] [PubMed] [Google Scholar]
- 9.Joiner MC, Van der Kogel AJ. The linear-quadratic approach to fractionation and calculation of isoeffect relationships. In: Steel GG, ed. Basic clinical radiobiology. New York, NY: Oxford University Press; 1997. pp. 106–12 [Google Scholar]
- 10.Yarnold J, Bentzen SM, Coles C, Haviland J. Hypofractionated whole-breast radiotherapy for women with early breast cancer: myths and realities. Int J Radiat Oncol Biol Phys 2011;79:1–9 10.1016/j.ijrobp.2010.08.035 [DOI] [PubMed] [Google Scholar]
- 11.Spyropoulou D, Kardamakis D. Review of hypofractionated radiotherapy for prostate cancer. ISRN Oncol 2012;2012:410892 10.5402/2012/410892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soyfer V, Corn BW, Kollender Y, Tempelhoff H, Meller I, Merimsky O. Radiation therapy for palliation of sarcoma metastases: a unique and uniform hypofractionation experience. Sarcoma 2010;2010:927972 10.1155/2010/927972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eilber FC, Rosen G, Eckardt J, Forscher C, Nelson SD, Selch M, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol 2001;19:3203–9 [DOI] [PubMed] [Google Scholar]
- 14.Ryan CW, Montag AG, Hosenpud JR, Samuels B, Hayden JB, Hung AY, et al. Histologic response of dose-intense chemotherapy with preoperative hypofractionated radiotherapy for patients with high-risk soft tissue sarcomas. Cancer 2008;112:2432–9 10.1002/cncr.23478 [DOI] [PubMed] [Google Scholar]
- 15.McKee MD, Liu DF, Brooks JJ, Gibbs JF, Driscoll DL, Kraybill WG. The prognostic significance of margin width for extremity and trunk sarcoma. J Surg Oncol 2004;85:68–76 10.1002/jso.20009 [DOI] [PubMed] [Google Scholar]
- 16.Morrow M, Harris JR, Schnitt SJ. Surgical margins in lumpectomy for breast cancer – bigger is not better. N Engl J Med 2012;367:79–82 10.1056/NEJMsb1202521 [DOI] [PubMed] [Google Scholar]