Abstract
Hyperhomocysteinemia (HHcy), a pathological condition characterized by an increase in plasma concentration of total homocysteine (Hcy), is recognized as a risk factor for several diseases. The transsulfuration pathway is the main metabolic fate of Hcy utilization, which requires the activity of cystathionine β-synthase (CBS). Our results showed the development of HHcy induced by psychological stress was mainly derived from a reduction of CBS activity in the liver, which was accompanied by a significant decrease in its mRNA level. It suggested that the hepatic CBS enzyme regulated by stress at the level of transcription would have a profound effect on circulating Hcy levels. The expression of Sp3, a negative factor for cbs transcription, obviously increased in hepatocytes nuclei of stressed rats, but Sp1 was not altered. It indicated that Sp3 was the key point of variations in cbs transcription caused by stress. Meanwhile, we detected that augmented plasma Hcy concentrations correlated with glucocordicoids (GCs) over-secretion in response to stress, and CBS mRNA levels were markedly lowered in GCs-treated rat hepatocytes. Further results found that glucocorticoids receptor (GR) expression in hepatocyte nuclei of stress rats and GR nuclear translocation ratio was increased, and the same results were proved by experiments in vitro, i.e., GR nuclear translocation and Sp3 expression was remarkably increased in GCs-treated hepatocytes. Moreover, results from ChIP suggested GCs enhanced the binding of GR to the regulatory region of the Sp3 promoter. These results indicated that GCs inhibit CBS transcription by up-regulating Sp3 in psychological stress-induced HHcy.
Keywords: Homocysteine, Cystathionine β-synthase, Psychological stress, Hyperhomocysteinemia, Glucocordicoids, Sp 1/3
Introduction
Homocysteine (Hcy), a cytotoxic sulfur-containing amino acid, is an intermediary metabolite in methionine/cysteine metabolism. The elevated plasma level of Hcy is termed hyperhomocysteinemia (HHcy), and regulation of Hcy is critically important for health maintenance since several diseases are related to HHcy (Diaz-Arrastia 2000; McCully 1996). Metabolism of Hcy has two main routes: (1) re-methylation, which salvages it back to the methionine cycle via cobalamin-dependent methionine synthase (MS)/5,10-methylenetetrahydrofolate reductase (MTHFR) or betaine homocysteine methyltransferase (BHMT); and (2) trans-sulfuration, which commits it to the synthesis of cysteine via the pyridoxal phosphate-dependent cystathionine β-synthase (CBS) and cystathionine γ-lyase (CGL) (Williams and Schalinske 2010). It is known that HHcy is caused either by genetic defects in the enzymes or by nutritional deficiency in vitamin cofactors involved in Hcy metabolism (Selhub 1999). But the aforementioned etiological factors made it difficult to elucidate the abnormal high incidence of HHcy.
Stress has been associated with various pathological conditions. In particular, human and animal studies have provided findings on mechanisms by which stress interferes with immune (Yudkin et al. 2000), neuroendocrine (Black and Garbutt 2002) and metabolic changes (de Oliveira et al. 2004) that may increase cardiovascular risk (Manuck et al. 1995) and psychiatric illness (Agid et al. 2000). It has been reported that plasma total Hcy levels increased after acute psychological stress in human and animal studies (Stoney 1999; de Oliveira et al. 2004). Our results also found that psychological stress could significantly elevate the plasma Hcy levels of rats. However, the pathological mechanisms of Hcy metabolic disorder induced by psychological stress remain largely unknown.
CBS occupies a crucial regulatory position between the methionine cycle and transsulfuration of Hcy metabolism. CBS (EC 4.2.1.22), the only enzyme for the catabolic removal of Hcy in mammals, is the rate-limiting step in the transsulfuration pathway and irreversibly converts Hcy to cysteine. Loss of CBS gene expression and/or function plays a very important role in human disease. For instance, severe CBS enzyme deficiency is the most common cause of homocystinuria, and partial deficiencies of CBS have been associated with HHcy (Mudd et al. 2001; Hamelet et al. 2007). Because of the key role of CBS in regulating plasma Hcy levels, there is a need for a greater understanding of the mechanisms and principles that govern its regulation.
Sp1/Sp3 belong to the Specificity Protein (Sp) transcription factor family of ubiquitously expressed factors, which activate or repress transcription of many genes in response to physiological and pathological stimuli (Suske et al. 2005). The CBS activity is regulated at the transcriptional level, and Sp1/Sp3 have been suggested as potential transactivators for the human CBS-1b promoter (Ge et al. 2001a, b; Maclean et al. 2004). The DNA binding domain of Sp1 and Sp3 share over 90 % DNA sequence homology, hence Sp1 and Sp3 recognize and associate with the same DNA element with similar affinity (Lin and Davie 2010). In most cases, an increase of the Sp1/Sp3 ratio has been correlated with the increased expression of response genes (Bouwman and Philipsen 2002). However, little is known regarding the role of Sp1/3 in transcriptional regulation of CBS expression in rodents subjected to stress. We suggest that Sp3 would be a good candidate because it could repress Sp1-mediated activation.
All stressors activate the hypothalamic–pituitary–adrenal (HPA) axis, and endogenic glucocordicoids (GCs) play an important role in the pathological impairments induced by stress (Chrousos and Gold 1992; Tsigosa and Chrousos 2002). GCs produce their effect on responsive cells by activating the glucocorticoids receptor (GR) to directly or indirectly regulate the transcription of target genes (Karin 1998). The number of genes per cell directly regulated by GCs is estimated to be between 10 and 100, but many genes are indirectly regulated through an interaction with other transcription factors (Ryuji et al. 2004). It has been shown that GCs can indirectly modulate the expression of Sp1-regulated genes (Marinovic et al. 2002).
The aim of the present study was to elucidate the molecular mechanism by which CBS expression is regulated in the rat liver during the psychological stress process. Our results demonstrated that psychological stress caused a reduction of CBS mRNA gene expression leading to a decrease in CBS enzyme activity, which resulted in HHcy. The activation of Sp3 accounted for decreased transcriptional activation of CBS gene. Further data revealed that GCs over-secretion induced by psychological stress inhibit CBS activity via up-regulating interaction between GR and Sp3. These findings would contribute to a better understanding on the factors that regulate plasma Hcy levels, and may potentially aid in preventing risks in a variety of conditions with abnormal Hcy metabolism.
Materials and methods
Rat model of psychological stress
The animal model of restraint stress was established as described previously (Zhao et al. 2009). This procedure mimics stress that is largely psychological in nature. Adult male Wistar rats weighing 180–200 g were divided randomly into a control and four stress groups (6 h, 1 week, 2 weeks and 3 weeks), eight rats in each group. Briefly, stress rats were placed individually into a specially built size-manipulable and adequately ventilated cabin for 6 h per day (from 9:00 am to 3:00 pm) for 21 consecutive days. The control rats were not disturbed during the 21-day period. Twenty-four hours after the last stress session, the rats were killed, and plasma and liver samples were collected and stored at −80 °C until assay.
All of the investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The ethics committees of the Beijing Institute of Basic Medical Sciences reviewed and approved our experimental procedures.
Hepatocytes model of stress
Hepatocytes were isolated from male pathogen-free rat liver by the collagenase perfusion technique (Berry et al. 1991). Rat hepatocytes were cultured in Iscove’s modified Dulbecco’s medium supplemented with fetal bovine serum (10 %), penicillin (100 units/ml), and streptomycin (100 μg/ml). Cells were grown to 75 % confluence under 5 % CO2 in a humidified incubator at 37 °C, and cell viability was at least 95 % in all cases. Cells were provided with fresh medium containing 0.5 % fetal bovine serum for 1 h before stress, and then were incubated with 10−6 M corticosterone (CORT) for 1, 6 and 12 h, respectively, with the aim of mimicking the exposure to psychosocial stress in rats. The CORT dose used in this experiment approximates to circulating levels of acute stress rats, that is, the peak concentration of CORT in plasma was about 1 × 10−6 M after 6 h stress.
Assay of plasma total homocysteine
Hcy was measured by high-performance liquid chromatography (HPLC), as previously reported by Durand et al. (1996). Briefly, 240 μl plasma was mixed with 60 μl of 2.5 mM acetyl-cysteine as internal standard. The Hcy-mixed disulfides or protein-bound Hcy in the plasma were treated with TCEP in order to reduce thiols and release them from plasma proteins. Proteins were then precipitated with 0.6 M cold perchloric acid containing 1 M EDTA, left at room temperature for 10 min after thorough mixing and centrifugation (2,000 × g, 10 min). Subsequently, the thiol compounds in the samples were derivatized with a thiol-specific fluorogenic reagent, SBD-F (pH 9.5). Complete derivatization of thiols was performed in a water bath at 60 °C during 60 min. Hcy in samples was analyzed using a HPLC system consisting of a Waters 2695 Liquid chromatograph, a Waters 2475 fluorescence detector (excitation 390 nm, emission 470 nm). Separation was carried out using a reverse phase column (Symmetry Shield™ RP18; 3.9 × 150 mm, 5 μm). Analysis was performed under isocratic concentration (0.08 M NaAc, 1 % methanol in water) at a flow rate of 0.8 ml/min for 15 min.
Determination of plasma corticosterone
Stress can activate the HPA axis and the sympathetic–adrenomedullary system, and the increased content of GCs and catecholamine in plasma are considered as a significant biological basis for evaluating stress load. The major component of GCs is CORT in rodents. Since the CORT/cortisol ratio in rat plasma is 50:1 constantly, plasma CORT content was calculated using cortisol content. Plasma cortisol concentration was measured by using 125I cortisol radioimmunoassay (RIA) kit (Beifang Bio-tech, Beijing, China) according to the manufacturer’s instructions.
Measurement of CBS activity
Liver tissue was homogenized in 0.1 mol/l sodium phosphate buffer (pH 7.4) containing phenylmethylsulfonyl fluoride (PMSF) for 1 min. The homogenate was centrifuged at 14,000 × g for 60 min at 4 °C and the supernatant was collected to enzyme assays. The CBS activity was determined by a classically described radioisotope assay using l-[U-14C]-serine (Amersham Pharmacia products) as the labeled substrate (Kraus 1987). One unit of CBS catalyzes the formation of 1 nmol of cystathionine in 1 h at 37 °C. Specific activity was expressed as units per mg of protein (nmol/h/mg pro).
The final concentration of the assay components in 0.2 ml of incubation mixture was: 100 mM Tris/HCl (pH 8.6), 0.25 mM pyridoxal-5′-phosphate, enzyme protein (0–100 μg), dialyzed bovine serum albumin added to a final protein concentration of 0.5 mg/ml, 10 mM serine adjusted with [14C]serine to a specific activity of 300 cpm/nmol, and 10 mM l-Hcy. Following a pre-incubation for 5 min at 37 °C, the reaction was started by the addition of the Hcy solution. After incubation for 30 to 60 min at 37 °C, an aliquot of the assay mixture was applied to Whatman No. 3 paper with drying in a hot air stream, and the [14C]cystathionine formed was separated from the [14C]serine by descending paper chromatography in 2-propanol/formic acid/H2O. Radioactivity in the region of marker l-cystathionine was determined in strips of the chromatogram in a standard toluene/PPO/POPOP mix in a liquid scintillation spectrometer (Rack Beta). An enzyme-free blank was subtracted. Crude extracts were assayed in the presence of 1 mM l-cystathionine to protect the radioactive product from degradation by cystathionase.
Analysis of CBS mRNA
Total RNA was extracted from hepatic cells by using Trizol reagent (Invitrogen, San Diego, CA, USA) and then analyzed by a reverse-transcription polymerase chain reaction (RT-PCR) and Northern blot method. RT-PCR (one step RNA PCR kit, TaKaRa, Tokyo, Japan) was performed with a primer specific for the cbs (forward: 5′-CATCCTGGAGATGGA-3′, reverse: 5′-ATGGAAGCCAGTCCTGACTTCTGAACTCT-3′) with the liver cell RNA as a template. The RT-PCR products were analyzed by electrophoresis on 2 % agarose gels.
In a Northern blot assay, an aliquot of total RNA (15 μg) sample was separated on a 1.5 % agarose gel containing formaldehyde and transferred to a nylon membrane by capillary blotting. Hybridization was done using the RT-PCR products that had been radiolabeled with [α-32p]-dCTP by random priming (random primer DNA labeling kit; TaKaRa). Autoradiograph of a hybridization blot was visualized on X-ray film, and its density was quantified using an image software package (ImageMaster VDS; Amersham Pharmacia Biotech, Uppsala, Sweden). The β-actin signals were used as internal controls.
Western blot analysis
For whole-cell protein extraction, liver tissues and hepatocytes were homogenized in radioimmunoprecipitation (RIPA) lysis buffer with a protease-inhibitor and centrifuged at 4 °C. For nuclear protein extracts, nuclei were isolated and lysed in nuclear extraction reagent. Protein concentrations were measured by the Bradford method with bovine serum albumin (BSA) as a standard.
Proteins were separated on an 8 % SDS (sodium dodecyl phosphate)-polyacrylamide gels using a mini-Protean cell (Bio-Rad, Hercules, CA, USA), and then transferred to polyvinylidine difluoride (PVDF) membranes (Millipore, Bedford, MA, USA) using a Trans-Blot SD cell. After blocking with 5 % fat-free milk, the membranes were first incubated with anti-CBS, anti-Sp1/Sp3 and anti-GR polyclonal antibody (1:1,000 dilution in blocking buffer; Santa Cruz Biotechnology, Santa Cruz, CA, USA), respectively, washed, and then incubated with horseradish peroxidase-conjugated secondary antibodies (1:1,000 dilution in blocking buffer; Zhongshan, Beijing, China). Immunoreactive bands were visualized on X-ray film using the chemiluminescence method. Band densities were quantified by densitometry, standardized to β-actin.
Preparation of nuclear extracts
Nuclear extracts from hepatocytes were prepared by standard methods, with slight modifications. The hepatic cells were digested with 0.25 % trypsin, harvested by centrifugation at 320 × g at 4 °C for 5 min, and washed in cold PBS (containing 1 mM Na3VO4, 5 mM NaF). Cell pellets were resuspended in 50 μl homogenate buffer (10 mM HEPES (pH7.8), 0.15 mM spermine, 0.75 mM spermidine, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 10 μg/ml pepstatin, 10 μg/ml leupeptin, 1 mM Na3VO4, 1 mM sodium pyrophosphate), placed on ice for 10 min, then 5 μl 75 % sucrose was added. The nuclei were pelleted by centrifugation at 13,000 × g at 4 °C for 5 min. Pellets were resuspended in 20–50 μl nuclear extract buffer containing 50 mM Tris–HCl (pH 7.9), 20 % glycerol, 10 % sucrose, 20 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, 2 mM DTT, 0.1 mM PMSF, and samples were incubated on a roller for 30 min at 4 °C followed by centrifugation at 13,200 × g for 20 min at 4 °C. The supernatants (nuclear extracts) were stored at −70 °C until analysis.
Chromatin immunoprecipitation (ChIP) assays
1 × 106 hepatocytes were cross-linked with 1 % formaldehyde at room temperature. After washing three times with ice-cold PBS, cell pellets were resuspended in 1 ml digestion buffer with enzyme inhibitors. The suspension was sonicated until cross-linked chromatin was sheared to an average DNA fragment length of 0.2–1.0 kb, and then centrifuged at 14,000 rpm for 10 min. The supernatant containing the sheared chromatin was used for ChIP reactions.
Immunoprecipitation was carried out with 2 μg per reaction antibody against GR (Santa Cruz Biotechnology), incubated overnight at 4 °C with rotation. The protein A-agarose (Roche)/antibody/chromatin complex was pelleted and washed successively for 5 min on a rotating platform with 1 ml each of: low-salt buffer, high-salt buffer, LiCl complex wash buffer and Tris/EDTA. 250 μl of freshly prepared elution buffer (1 % SDS, 0.1 M NaHCO3) was added to the pelleted protein A–agarose complex, vortexed briefly to mix and incubated at room temperature for 15 min with rotation. The tubes were centrifuged at 1,000 rpm for 1 min at room temperature and the supernatant fraction was collected. The elution step was repeated and the eluates were combined. The protein–DNA cross-links were reversed, by adding 20 μl of 5 M NaCl to the combined eluates and heating at 65 °C for 4 h. Then, 10 μl of 0.5 M EDTA, 20 μl 1 M Tris–HCl, pH 6.5 and 2 μl of 10 mg/ml Proteinase K was added to the combined eluates and incubated for 1 h at 45 °C. After the addition of 3 μl glycogen (2 μg/μl), DNA was recovered by phenol/chloroform extraction and ethanol precipitation and re-dissolved in Tris/EDTA, pH 8.4 for use in PCR analyses.
PCR amplification of fragments containing glucocorticoid response elements (GREs)-binding site of the Sp1/Sp3 promoter region was performed with the following primers: Sp1 (forward: 5′-CCA TGA GCG ACC AAG ATC AC-3′, reverse: 5′-GGA GTT GTT GCT GTT CTC AT-3′) and Sp3 (forward: 5′-CTA TGA GGT TGG AGC CCA GG-3′, reverse: 5′-ACA GAA CAC ATG AAA CTC AA −3′). Amplified segments for Sp1 (254 bp) and Sp3 (458 bp) were analysed using 2 % agarose gels in TAE buffer.
Statistics
All data are presented as means ± SD. Statistical analysis was performed by Student’s t-test and p < 0.05 was considered statistically significant.
Results
Elevation of plasma Hcy levels in psychologically stressed rats
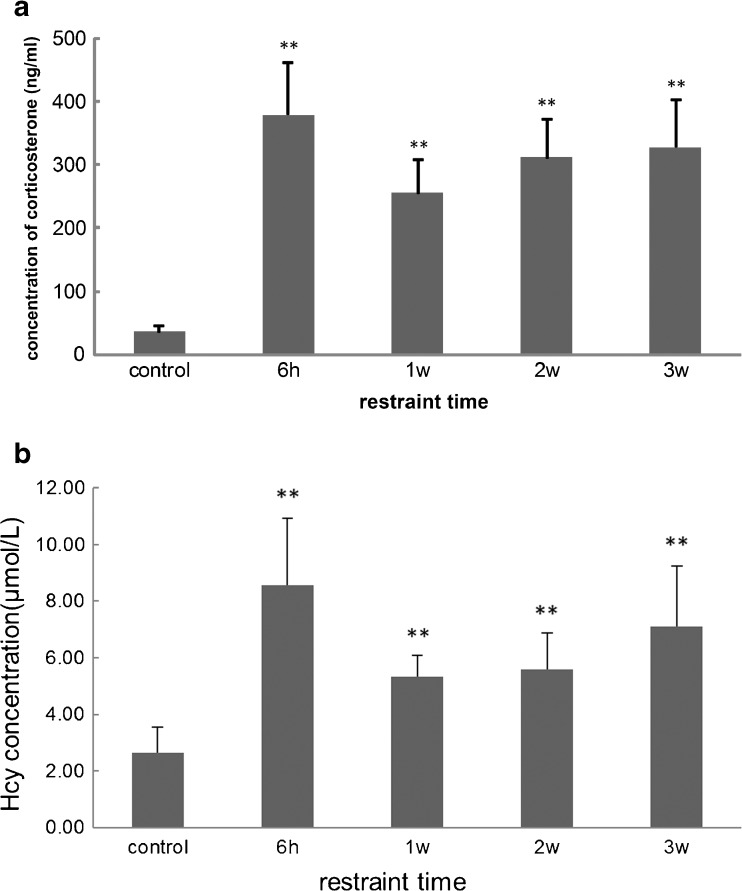
As an essential stress hormone, the CORT level in rat plasma is a sensitive index reflecting the stress load. The results showed that the plasma CORT concentration increased significantly after psychological stress (Fig. 1a), and the peak concentration of CORT was observed in the acute stress group (stress for 6 h), high plasma CORT levels were maintained during chronic stress (stress for 1–3 weeks), which indicated that the psychological stress animal model had been established.
Fig. 1.
Effect of restraint stress on corticosterone and Hcy concentrations in rat plasma. a Corticosterone concentration in rat plasma was increased significantly after restraint stress. Compared to control, the peak was observed in the group of acute stress for 6 h (379.1 ± 81.3 vs. 36.2 ± 9.8 ng/ml), and higher levels were maintained during chronic stress for 3 weeks (255 ± 53.3 ng/ml for 1-week stress, 310.9 ± 59.7 ng/ml for 2-week stress and 327.7 ± 75.1 ng/ml for 3-week stress, respectively). b The plasma Hcy levels in rats exposed to stress were markedly increased. The peak concentration of Hcy occurred in the acute stress group, and increased by 3.3-fold compared with the control (8.57 ± 2.37 vs. 2.64 ± 0.91 μmol/l). In the chronic stress groups, Hcy levels gradually increased with stress time prolongation, resulting in an approximately 2.7-fold increase (7.11 ± 2.14 μmol/l) after psychological stress 3 weeks. (n = 8, **p < 0.01 compared with control)
As shown in Fig. 1b, plasma Hcy levels of rats under stress were markedly increased compared to control. The peak concentration of Hcy occurred in the acute stress group, and increased by 3.3-fold compared with the control (8.57 ± 2.37 vs. 2.64 ± 0.91 μmol/l). In the chronic stress groups, Hcy levels gradually increased with stress time prolongation, an approximately 2.7-fold increase (7.11 ± 2.14 μmol/l) after psychological stress lasting 3 weeks. This indicates that restraint stress may lead to Hcy accumulation in rat plasma.
Disorder of CBS in stress-induced HHcy rats
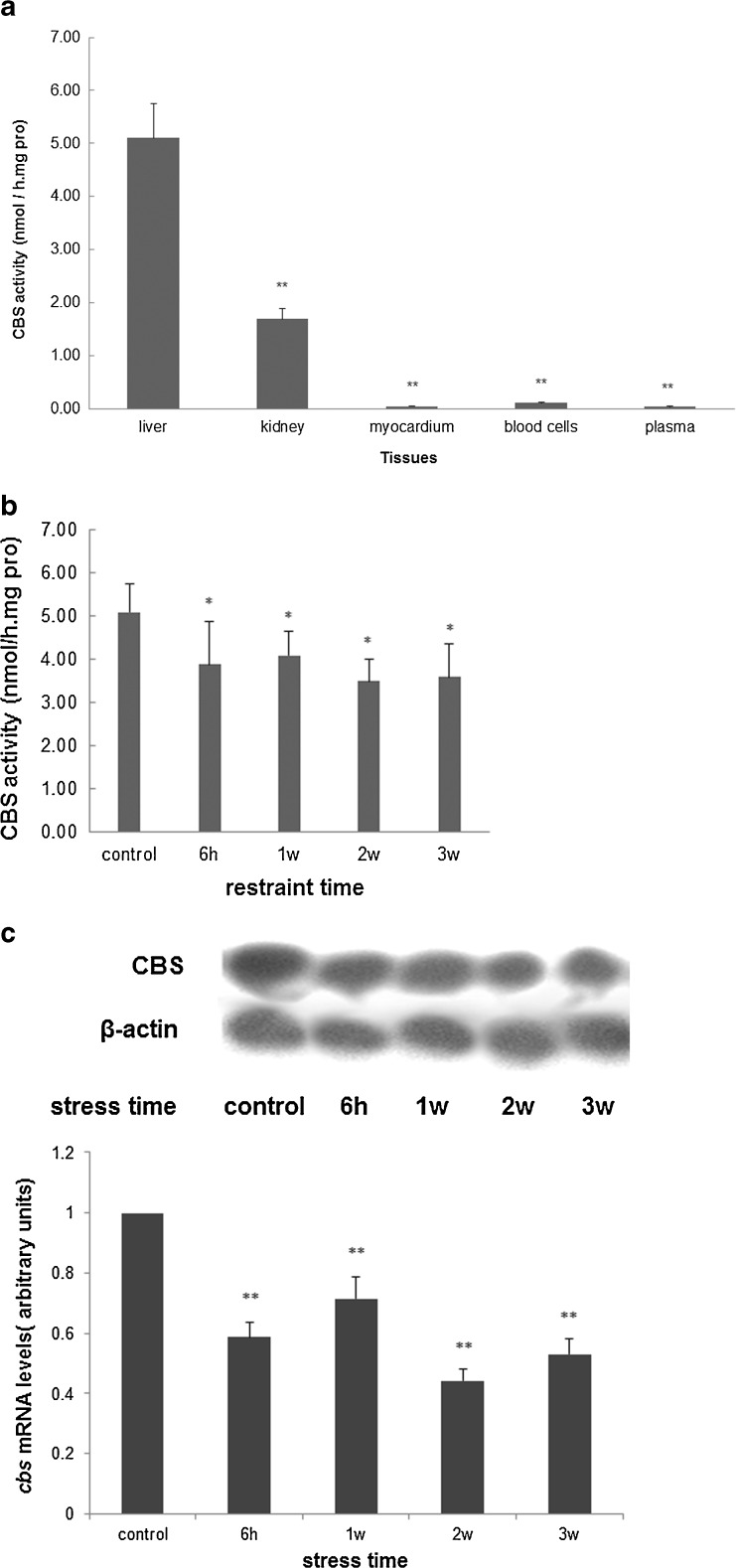
To examine whether the elevation of Hcy levels in the stressed rat could be attributed to a decrease in CBS enzyme activity, the CBS enzyme activity in blood, hearts, livers and kidneys from control and stressed rats was measured (Fig. 2a). It was found that both plasma and blood cells showed almost undetectable CBS activity, as did hearts. The highest CBS enzyme activity was found in normal rat livers, and the next highest was found in kidney. But compared to control, it was decreased in liver after stress, and slightly elevated in kidney inversely. CBS enzyme activity in livers decreased during the restraint stress process, and decreased to 70.6 % of the control after 3 weeks stress (3.6 ± 0.76 vs. 5.1 ± 0.65 nmol/h/mg pro; Fig. 2b). Meanwhile, results from Northern blot demonstrated that CBS mRNA levels were markedly lowered in stressed rat livers compared with control, to 44.1 % of the control (Fig. 2c). These results showed that the change in hepatic CBS mRNA levels was in parallel with the enzyme activity, and inhibition of CBS activity in rat liver may be regulated at the transcriptional level.
Fig. 2.
Inhibition of CBS in stress-induced HHcy Rats. a CBS enzyme activity in blood, hearts, livers and kidneys from control and stressed rats was measured. It was found that both plasma and blood cells showed almost undetectable CBS activity, as did hearts. The highest CBS enzyme activity was found in normal rat livers, and the next highest in rat kidney (n = 8, **p < 0.01 compared with liver). b CBS activity in livers of rats subjected to stress was decreased. After stress for 3 weeks, it decreased to 70.6 % of the control (3.6 ± 0.76 vs. 5.1 ± 0.65 units; n = 8, *p < 0.05 compared with control). c The result from Northern blot showed that CBS mRNA levels were markedly lowered in stressed rat livers, to 44.1 % of the control. β-Actin was used as an internal control, and the change ratio is normalized by β-actin based on OD value of bands (n = 3, **p < 0.01 compared with control)
Changes of Sp1/Sp3 in stress-induced HHcy rats
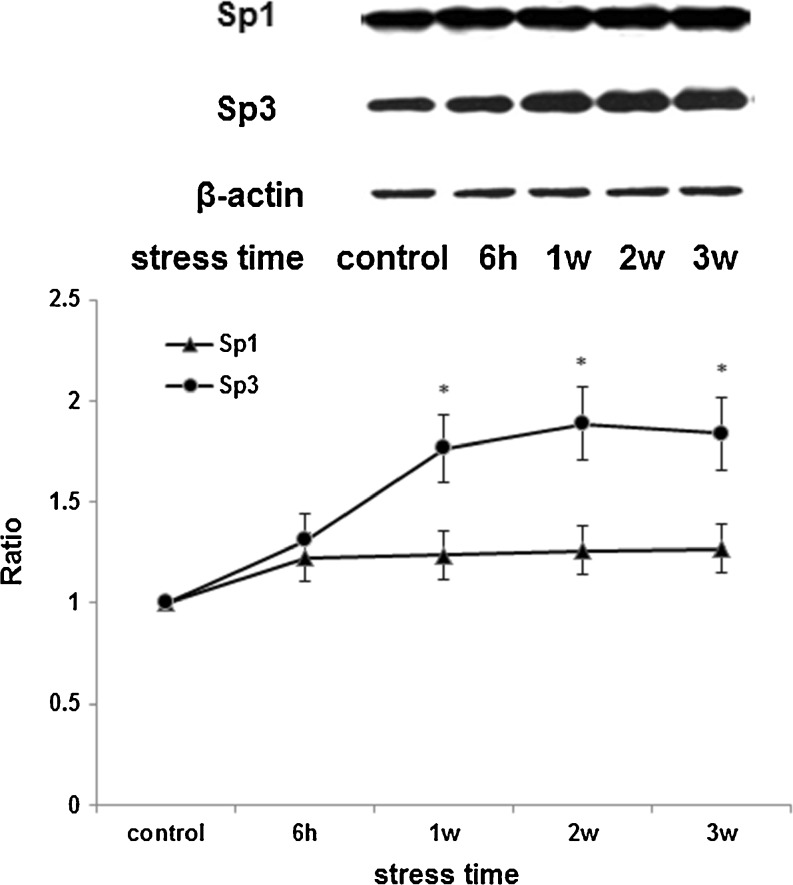
Levels of Sp1 and Sp3 expression are critical for regulation of CBS, and thus to address the regulatory mechanism of CBS expression, we next examined Sp1 and Sp3 levels in the nuclei of rat livers during stress. As shown in Fig. 3, the expression of Sp3, a negative factor for cbs transcription, was found to be increased in hepatocytes nuclei of stressed rats, whereas the Sp1 protein level remained unchanged after stress, a positive transcription factor for CBS. A change of Sp1/Sp3 levels and ratios demonstrates lower levels of Sp1 and higher Sp3 expression (decreased Sp1/Sp3 ratios) in stressed liver cells when compared with control. The results suggested that restraint-stress inhibits CBS gene promoters by up-regulating Sp3, thereby strengthening the associated transcriptional repression.
Fig. 3.
Changes of Sp1 and Sp3 expression in the nuclei of rat livers during restraint stress. The expression of Sp3, a negative factor for cbs transcription, was found to be increased in hepatocytes nuclei of stressed rats, the ratios (stress/control) of relative densitometry were 1.31, 1.76, 1.89 and 1.84 for stress lasting 6 h, 1 weeks, 2 weeks and 3 weeks, respectively. But Sp1, a positive transcription factor for cbs, remained almost unchanged after stress. That is Sp1/Sp3 ratios decreased in stressed rat liver cells when compared with control. β-Actin was used as an internal control, and the changed ratio of proteins is normalized by β-actin based on OD value of proteins bands. Graphical representations of Sp1 and Sp3 are shown in the bottom panel with changes in expression over time (n = 3, *, p < 0.05 compared with control)
Regulation of CBS expression by GCs during stress
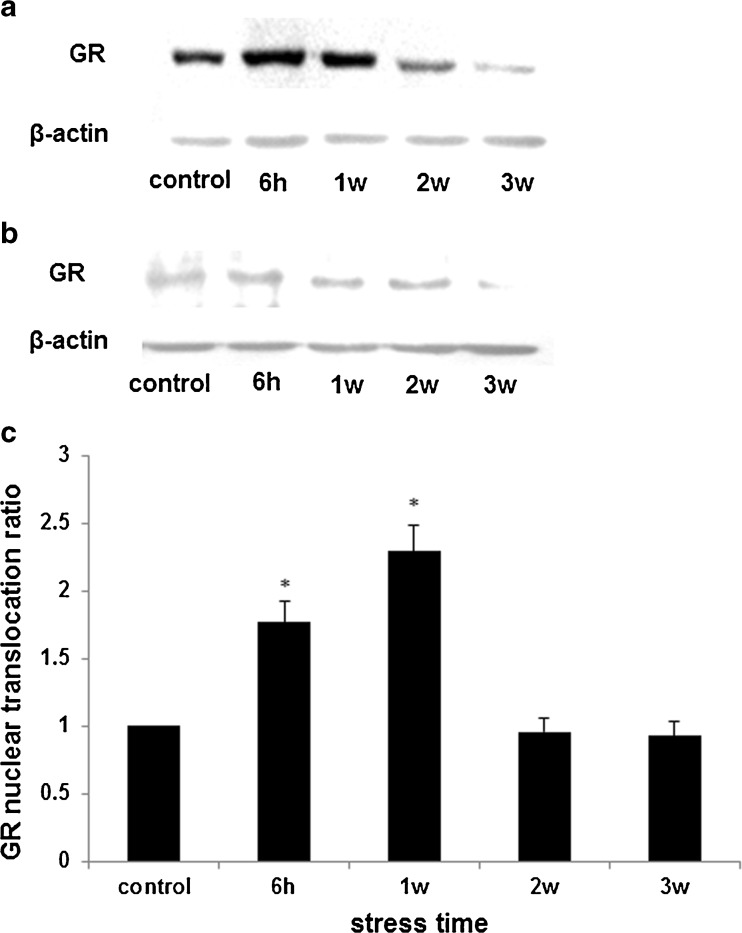
The degree of GR nuclear localization is a critical factor in determining the level of GR function. Our results found that GR expression in hepatocyte nuclei of restrained rats was increased, especially in groups of stress for 6 h and 1 week (Fig. 4a), which was associated with GR depletion from the cytoplasm (Fig. 4b). This demonstrated that psychological stress could induce GR nuclear translocation. Graphical representations of GR nuclear translocation showed the changes in the ratio over time (Fig. 4c).
Fig. 4.
Changes of GR nuclear translocation in stress-induced HHcy. Glucocorticoids bind to cytoplasmic GR, which then translocate to the nucleus where they regulate gene expression. GR expression in hepatocyte nuclei of restrained rats was increased, especially in groups of stress for 6 h and 1 week, about 1.45-fold and 1.48-fold vs. control (a), which is associated with GR depletion from the cytoplasm (b). There is a significant increase in GR nuclear translocation after stress 6 h and 1 week, and it returned to the normal level after 2 weeks of stress (c). β-Actin was used as an internal control, and the change ratio of proteins is normalized by β-actin based on OD value of proteins bands (n = 3, *p < 0.05 compare with control)
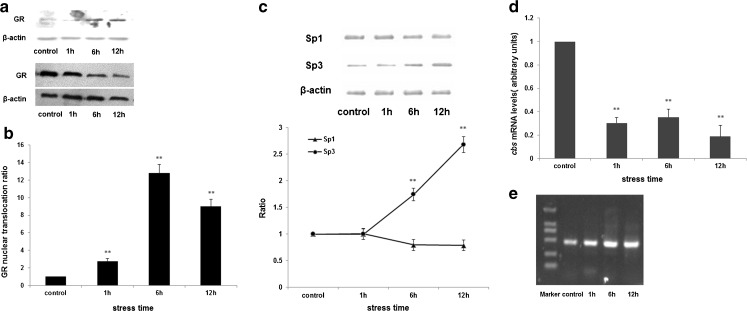
To determine whether GCs exerted control over the transcription of the CBS gene, nuclear ChIP assays were performed on isolated nuclei from hepatic cells that had been incubated with 10−6 M CORT for 1, 6 and 12 h. The results showed that the expression of GR was increased in nuclei of hepatic cells with CORT treatment, and decreased to about 31 % in the hepatic cytoplasm after treatment with CORT for 12 h (Fig. 5a). Compared with control, GR nuclear translocation was remarkably increased after CORT stress (Fig. 5b).
Fig. 5.
Regulation of CBS activity by GC during stress. To determine whether glucocorticoids exerted direct control over cbs transcription, we examined hepatocytes that had been incubated with10−6 mol/l CORT for 1, 6 and 12 h. The expression of GR was increased in nuclei of hepatic cells with CORT treatment (a, top), and was decreased to about 31 % in the hepatic cytoplasm after treatment with CORT for 12 h (a, bottom). Compared with control, GR nuclear translocation was remarkably increased after CORT stress (b). c The level of Sp3 expression in nuclei of GCs-treated hepatocytes was elevated, and Sp1 expression was slightly reduced. As a result, the ratio of Sp1/Sp3 was decreased. Graphical representations of Sp1 and Sp3 are shown in the bottom panel with changes in expression over time. d CBS mRNA levels were markedly lowered in GCs-treated hepatocytes compared with control, to 19 % of the control after CORT treatment for 12 h. e The results from CHIP and PCR showed the numbers of the Sp3 specific fragments (458 bp) were increased after CORT treatment, but the change of Sp1(254 bp) was not observed (data not shown). It suggested that GCs enhance the binding of GR and the regulatory region (GREs) of Sp3 promoter and have no effect on Sp1. β-Actin was used as an internal control, and the change ratio of proteins is normalized by β-actin based on OD value of proteins bands (n = 3, **p < 0.01 compare with control)
Meanwhile, as shown in Fig. 5c, the level of Sp3 expression in nuclei of CORT-treated hepatocytes was elevated and a change of Sp1 expression was not detected. As a result, Sp3 activity was increased, while Sp1 activity was decreased, which in turn led to the decrease of cbs transcription. CBS mRNA levels were markedly lowered in CORT-treated hepatocytes compared with control, to 19 % of the control after CORT treatment for 12 h (Fig. 5d).
Furthermore, the sequence analysis showed that the upstream regulatory region of the Sp1/Sp3 promoter contain GREs that are responsible for GCs inducibility. The results from ChIP and PCR showed that the amounts of the Sp3 specific fragment were increased after CORT treatment (Fig. 5e), but the change of Sp1 was not observed (data not shown). This indicated that GCs significantly enhanced the amount of GR bound to the Sp3 promoter, but had no effect on Sp1 promoter activity.
Discussion
Hcy accumulated in psychologically stressed rat plasma
Hcy is a thiol amino-acid synthesized during the metabolic conversion of methionine to cysteine. Once generated, Hcy is either metabolized to cysteine via the transsulfuration pathway or remethylated to methionine via the remethylation pathway. When excess Hcy is produced in the body and is not readily converted into methionine or cysteine, it is excreted from the tightly regulated intracellular environment into the blood as cytotoxic. Hcy levels rise beyond normal levels (i.e., HHcy) and lead to adverse health outcomes (Troen et al. 2008). Usually, Hcy in rat plasma was diet-induced, i.e., by feeding high-methionine diet (Morita et al. 2001). However, some researchers reported their results after a rat was treated using different acute stress manipulations, including restraint stress, swimming and cold, and found that restraint stress was the only type of stress that altered Hcy concentrations in rats (de Souzza et al. 2006; de Oliveira et al. 2004). Their articles indicate the specific relationship between restraint stress and the increase of Hcy levels. Restraint is a model that mainly simulates psychological stress in rats. Furthermore, similar results were reported by Stoney (1999), who demonstrated a significant elevation (+7 %) of plasma Hcy in women in response to psychological stress (e.g., when performing mental arithmetic calculations). In this study, we can see that Hcy concentration was increased more than 3-fold after 3 weeks of restraint stress, and our previous study detected that plasma Hcy levels may be 2–4 times the normal in a chronic restraint stress process (Chen et al. 2009). These results indicate that restraint stress may lead to Hcy accumulation in rat plasma. Nowadays, there are increasing numbers of psychological stress-related diseases, and more attention should be paid to the pathogenesis and prevention of stress-induced injury.
Inhibition of CBS activity in psychologically stressed rat livers
CBS enzyme activity plays an important role in determining plasma Hcy levels. Our results showed some difference in CBS activity in several tissues in normal subjects. Little CBS activity was seen in the cardiovascular system, including heart and blood, which implied that the cardiovascular system was the key target, but not the regulative site, of HHcy. The limited metabolic capacity for Hcy in the cardiovascular system might be linked to the injury of Hcy to the cardiovascular system. The liver showed the highest catabolic capacity for Hcy and kidney the second highest. After stress, CBS enzyme activity in rat liver was clearly reduced, whereas (although less than in liver) it was increased in the kidney of stressed rats. This may be due to the fact that the kidney is an important organ in the removal of Hcy, and it could be taken as a compensatory effect for elevated Hcy levels in plasma. Our experiments indicate that the liver is indeed a key organ of Hcy metabolism and potentially contributes to much of the plasma Hcy levels. In many individuals with liver disease, Hcy levels can rise beyond normal levels (Borgia et al. 2008). Our current findings are in accordance with an earlier study that reported the basal expression of CBS is fairly strong in rat liver cells (Jacobs et al. 1998).
The human CBS gene has been localized to chromosome 21 (21q22.3) and is overexpressed in the trisomy 21 of Down Syndrome patients (Pogribna et al. 2001), who have lower than normal plasma Hcy. Similarly, plasma Hcy levels are 40 times higher in CBS−/− mice than in wild-type mice (Watanabe et al. 1995), and increased enzyme activity in transgenic mice decreases serum Hcy levels (Wang et al. 2004). Heterozygous CBS+/− mice have a 50 % reduction in CBS expression and twice the normal Hcy levels (Chadefaux et al. 1988). Therefore, CBS expression plays an important role in determining plasma Hcy levels. Our study has revealed that the activity of CBS is markedly reduced in rat livers subjected to restraint stress, and further study found that CBS mRNA abundance in livers of stressed rats was also decreased. It is likely that the effect of restraint stress on CBS activity is regulated at the level of gene transcription, since hepatic mRNA levels for CBS were in parallel with the enzyme activity. Yamamoto et al. (1996) have shown that a high casein diet-induced increase in CBS activity was followed by an increase in CBS mRNA levels in rat liver.
These results suggest that the disorder of transsulfuration metabolism was the main regulatory pathway in restraint stress-induced HHcy, in which liver acted as the predominant locus. The development of HHcy was mainly derived from a reduction in hepatic CBS activity which was accompanied by a significant decrease in its mRNA level. However, the molecular controls of CBS expression need to be further investigated.
Regulation of CBS expression by GCs over-secretion during psychological stress
It has been confirmed that loss of CBS gene expression and/or function has been adversely associated with mammal health and disease. In light of our findings, the hepatic transsulfuration pathway regulated by stress at the level of transcription would have a profound effect on circulating levels of Hcy. Studies have demonstrated that the critical cis-elements that regulate CBS basal transcription from the major CBS-1b promoter are 3 GC boxes, an inverted CAAT-box and an E-box, the correlative trans-acting factors are Sp1/Sp3, upstream stimulatory factor 1 (USF-1) and nuclear factor (NF)-Y/NF-1-like factor, respectively. Sp1 has been reported to play a dominant role in the regulation of CBS (Ge et al. 2001a, b). Studies found that co-expression of Sp1 and Sp3 occurs and competes for the same binding sites in vivo, whereas Sp3 remains inactive or acts only as a very weak activator, which represses Sp1-mediated activation, so that the relative abundance of Sp1 and Sp3 should allow regulation of gene activities (Suske 1999; Sade et al. 2009; Hagen et al. 1994).
Our results showed that the expression of Sp3 increased in hepatocytes of stressed rats, but Sp1 was not altered after stress. This suggests that the relative level of Sp1/Sp3 was the key point of variation in CBS transcription caused by psychological stress, and Sp3 decreased the activity of Sp1 in stressed hepatocytes. Ge et al. (2003) demonstrated that the differential binding of Sp1/Sp3 to the CBS-1b promoter in CMK versus CMS cells may, in part, explain differences in the levels of CBS transcripts and CBS-1b promoter activity between the lines. Attenuation of Sp1-dependent transactivation of GC-rich promoters has been reported in some experiments by interactions of Sp1 and Sp3. For example, it was reported that basal expression of the 15-lipoxygenase-2 gene in human prostate epithelial cells is dependent on interactions of Sp1 with GC-rich sites, whereas Sp3 decreases activity from the same sites (Tang et al. 2004). Expression of the human activator protein 2c gene in breast tumor cells is also regulated by Sp1 (induction) and Sp3 (inhibition) interactions with GC-rich sites, and the cellular Sp1/Sp3 ratios is a determinant factor in the expression of this gene in breast cancer cell lines (Hazleton et al. 2003). These results also supported the outcome of our experiments, and the role of Sp3 in regulating CBS promoter during psychological stress may be dominant and indispensable, so that Sp3 was negative for CBS activity.
How does psychological stress regulate Sp-dependent transcription of the CBS promoter? Our study demonstrated that increased plasma Hcy concentrations correlated with changes in GCs levels in response to restraint stress. Moreover, CBS mRNA levels were markedly lowered in GCs-treated rat hepatocytes compared with control. Thus, GCs alterations do seem to participate in Hcy regulation. GCs play an important role in the control of hepatic genes encoding regulatory enzymes of intermediary metabolism, including those enzymes involved in amino acid metabolism. However, some results suggested that GCs alter plasma Hcy concentrations by stimulating CBS expression in diabetic rats (Ratnam et al. 2002), and GCs could increase the level of CBS enzyme activity in rat hepatoma variants (Goss 1986). The reasons for these inconsistencies are not clear, and may be brought about by cell-specific manner and/or selectivity. Further work is required to investigate the exact mechanism by which GCs represses the expression of CBS.
It has been described that GCs can modulate the expression of Sp1-regulated genes (Marinovic et al. 2002). The mechanisms whereby GCs could modulate the transcription of genes involve interactions between the GR and other transcription factors. GCs bind to cytoplasmic GR, which then translocate into the nucleus, where it binds as homodimer to GREs located in the promoter region of target genes and regulates gene expression. Our results found that GR expression in hepatocyte nuclei of restraint rats was increased, and there was a significant increase in GR nuclear translocation after stress. The same result was demonstrated by experiments in vitro, i.e., GR nuclear translocation was remarkably increased and Sp3 expression and activity were elevated in nuclei of GCs-treated hepatocytes. In addition, the sequence analysis of Sp1/Sp3 showed that the upstream regulatory regions of Sp1/Sp3 promoter all contain GREs that are responsible for GCs inducibility. Furthermore, the results from ChIP and PCR showed that the number of the Sp3 specific fragments was increased after GCs treatment, which suggested that GCs enhanced the binding of GR and the regulatory region of Sp3 promoter, and thus up-regulated the Sp3 level.
In conclusion, our data indicate that psychological stress could induce HHcy through inhibition of the transsulfuration pathway of Hcy metabolism in the rat liver. The indirect control of GCs on CBS expression occurs at the transcriptional level, and the GCs-GR mediated pathway plays an important role in Sp3-dependent CBS activity regulation. Understanding the factors that regulate plasma Hcy levels may potentially aid in preventing risks in a variety of conditions, including stress-related disorders.
Acknowledgements
This work was supported by the National Program on Key Basic Research Project of China (2011CB505106) and the National Natural Science Foundation of China (31071022).
Abbreviations
- HHcy
Hyperhomocysteinemia
- Hcy
Homocysteine
- CBS
Cystathionine β-synthase
- Sp
Specificity Protein
- GCs
Glucocordicoids
- GR
Glucocorticoids receptor
- GREs
Glucocorticoid response elements
Footnotes
Zhao Yun and Wu Shuqing contributed equally to this work.
References
- Agid O, Kohn Y, Lerer B. Environmental stress and psychiatric illness. Biomed Pharmacother. 2000;54:135–141. doi: 10.1016/S0753-3322(00)89046-0. [DOI] [PubMed] [Google Scholar]
- Berry MN, Edwards AM, Barritt GJ (1991) High yield preparation of isolated hepatocytes from rat liver. In: Burdon RH and van Knippenburg PH (ed) Laboratory Techniques in Biochemistry and Molecular Biology, Elsevier, Oxford, 21:44–57
- Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52:1–23. doi: 10.1016/S0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Borgia G, Gentile I, Fortunato G, Borrelli F, Borelli S, de Caterina M, Taranto MDD, Simone M, Borgia F, Viola C, Reynaud L, Cerini R, Sacchetti L. Homocysteine levels and sustained virological response to PEGylated-interferon alpha2b plus ribavirin therapy for chronic hepatitis C: a prospective study. Liver Int. 2008;29:248–252. doi: 10.1111/j.1478-3231.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol. 2002;195:27–38. doi: 10.1016/S0303-7207(02)00221-6. [DOI] [PubMed] [Google Scholar]
- Chadefaux B, Ceballos I, Hamet M, Coude M, Poissonnier M, Kamoun P, Allard D. Is absence of atheroma in Down syndrome due to decreased homocysteine levels? Lancet. 1988;2:741. doi: 10.1016/S0140-6736(88)90211-5. [DOI] [PubMed] [Google Scholar]
- Chen M, Wang Y, Zhao Y, Wang L, Gong J, Lei W, Gao X, Yang Z, Qian L. Dynamic proteomic and metabonomic analysis reveal dysfunction and subclinical injury in rat liver during restraint stress. BBA-Proteins Proteom. 2009;1794:1751–1765. doi: 10.1016/j.bbapap.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. doi: 10.1001/jama.1992.03480090092034. [DOI] [PubMed] [Google Scholar]
- de Oliveira AC, Suchecki D, Cohen S, D'Almeida V. Acute stressor-selective effect on total plasma homocysteine concentration in rats. Pharmacol Biochem Behav. 2004;77:269–273. doi: 10.1016/j.pbb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- de Souzza F, Rodrigues M, Tufik S, Nobrega J, D'Almeida V. Acute stressor-selective effects on homocysteine metabolism and oxidative stress parameters in female rats. Pharmacol Biochem Behav. 2006;85:400–407. doi: 10.1016/j.pbb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Diaz-Arrastia R. Homocysteine and neurologic disease. Arch Neurol. 2000;57:1422–1427. doi: 10.1001/archneur.57.10.1422. [DOI] [PubMed] [Google Scholar]
- Durand P, Fortin LJ, Lussier-Cancan S. Hyperhomocysteinemia induced by folic acid deficiency and methionine load-application of a modified HPLC method. Clinica Chimica Acta. 1996;252:83–93. doi: 10.1016/0009-8981(96)06325-5. [DOI] [PubMed] [Google Scholar]
- Ge Y, Konrad MA, Matherly LH, Taub JW. Transcriptional regulation of the human cystathionine beta-synthase-1b basal promoter: synergistic transactivation by transcription factors NF-Y and Sp1/Sp3. Biochem J. 2001;357:97–105. doi: 10.1042/0264-6021:3570097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Matherly LH, Taub JW. Transcriptional regulation of cell-specific expression of the human cystathionine-beta-synthase gene by differential binding of Sp1/Sp3 to the -1B promoter. J Biol Chem. 2001;276:43570–43579. doi: 10.1074/jbc.M104930200. [DOI] [PubMed] [Google Scholar]
- Ge Y, Jensen TL, Matherly LH, Taub JW. Transcriptional regulation of the cystathionine-beta-synthase gene in Down syndrome and non-Down syndrome megakaryocytic leukemia cell lines. Blood. 2003;101:1551–1557. doi: 10.1182/blood-2002-07-2337. [DOI] [PubMed] [Google Scholar]
- Goss SJ. Characterization of cystathionine synthase as a selectable, liver-specific trait in rat hepatomas. J Cell Sci. 1986;82:309–320. doi: 10.1242/jcs.82.1.309. [DOI] [PubMed] [Google Scholar]
- Hagen G, Muller S, Beato M, Suske G. Sp1-mediated transcriptional activation is repressed by Sp3. EMBO J. 1994;13:3843–3851. doi: 10.1002/j.1460-2075.1994.tb06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelet J, Demuth K, Paul JL, Delabar JM, Janel N. Hyperhomocysteinemia due to cystathionine beta synthase deficiency induces dysregulation of genes involved in hepatic lipid homeostasis in mice. J Hepatol. 2007;46:151–159. doi: 10.1016/j.jhep.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Hazleton MD, Ibbitt JC, Hurst HC. Characterization of the human activator protein-2c (AP-2c) gene: control of expression by Sp1/Sp3 in breast tumour cells. Biochem J. 2003;373:925–932. doi: 10.1042/BJ20030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RL, House JD, Brosnan ME, Brosnan JT. Effects of streptozotocin-induced diabetes and of insulin treatment on homocysteine metabolism in the rat. Diabetes. 1998;47:1967–1970. doi: 10.2337/diabetes.47.12.1967. [DOI] [PubMed] [Google Scholar]
- Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell. 1998;93:487–490. doi: 10.1016/S0092-8674(00)81177-0. [DOI] [PubMed] [Google Scholar]
- Kraus JP. Cystathionine β-synthase (human) Meth Enzymol. 1987;143:388–394. doi: 10.1016/0076-6879(87)43068-1. [DOI] [PubMed] [Google Scholar]
- Lin L, Davie JR. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann Anat. 2010;192:275–283. doi: 10.1016/j.aanat.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Maclean KN, Kraus E, Kraus JP. The dominant role of Sp1 in Regulating the cystathionine β-synthase-1a and -1b promoters facilitates potential tissue-specific regulation by Kruppel-like factors. J Biol Chem. 2004;279:8558–8566. doi: 10.1074/jbc.M310211200. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Kaplan JR, Williams JK. The pathogenicity of behavior and its neuroendocrine mediation: an example from coronary artery disease. Psychosom Med. 1995;57:275–283. doi: 10.1097/00006842-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Marinovic AC, Zheng B, Mitch WE, Price SR. Ubiquitin (UbC) expression in muscle cells is increased by glucocorticoids through a mechanism involving Sp1 and MEK1. J Biol Chem. 2002;277:16673–16681. doi: 10.1074/jbc.M200501200. [DOI] [PubMed] [Google Scholar]
- McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2:386–389. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- Morita H, Kurihara H, Yoshida S, Saito Y, Shindo T, Oh-Hashi Y, Kurihara Y, Yazaki Y, Nagai R. Diet-induced hyperhomocysteinemia exacerbates neointima formation in rat carotid arteries after balloon injury. Circulation. 2001;103:133–139. doi: 10.1161/01.CIR.103.1.133. [DOI] [PubMed] [Google Scholar]
- Mudd SH, Levy HL, Kraus JP. Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8. New York: McGraw-Hill; 2001. pp. 2007–2056. [Google Scholar]
- Pogribna M, Melnyk S, Pogribny I, Chango A, Yi P, James SJ. Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am J Hum Genet. 2001;69:88–95. doi: 10.1086/321262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam S, Maclean KN, Jacobs RL, Brosnan ME, Kraus JP, Brosnan JT. Hormonal regulation of cystathionine β-synthase expression in liver. J Biol Chem. 2002;277:42912–42918. doi: 10.1074/jbc.M206588200. [DOI] [PubMed] [Google Scholar]
- Ryuji H, Hiroo W, Ito K, Ian MA. Effects of glucocorticoids on gene transcription. Eur J Pharmacol. 2004;500:51–62. doi: 10.1016/j.ejphar.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Sade H, Holloway K, Romero IA, Male D. Transcriptional control of occludin expression in vascular endothelia: regulation by Sp3 and YY1. Biochim Biophys Acta. 2009;1789:175–184. doi: 10.1016/j.bbagrm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- Stoney CM. Plasma homocysteine levels increase in women during psychological stress. Life Sci. 1999;64:2359–2365. doi: 10.1016/S0024-3205(99)00189-7. [DOI] [PubMed] [Google Scholar]
- Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/S0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: bring in the family. Genomics. 2005;85:551–556. doi: 10.1016/j.ygeno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Tang S, Bhatia B, Zhou J, et al. Evidence that Sp1 positively and Sp3 negatively regulate and androgen does not directly regulate functional tumor suppressor 15-lipoxygenase 2 (15-LOX2) gene expression in normal human prostate epithelial cells. Oncogene. 2004;23:6942–6953. doi: 10.1038/sj.onc.1207913. [DOI] [PubMed] [Google Scholar]
- Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci USA. 2008;105:12474–12479. doi: 10.1073/pnas.0805350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigosa C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–887. doi: 10.1016/S0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Wang L, Jhee KH, Hua X, DiBello PM, Jacobsen DW, Kruger WD. Modulation of cystathionine beta-synthase level regulates total serum homocysteine in mice. Circ Res. 2004;94:1318–1324. doi: 10.1161/01.RES.0000129182.46440.4a. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KT, Schalinske KL. Homocysteine metabolism and its relation to health and disease. Biofactors. 2010;36:19–24. doi: 10.1002/biof.71. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Tanaka T, Noguchi T. The effect of a high-protein diet on cystathionine b-synthase activity and its transcript levels in rat liver. J Nutr Sci Vitaminol. 1996;42:589–593. doi: 10.3177/jnsv.42.589. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/S0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Jun X, Gong JB, Qian LJ. l-Type calcium channel current up-regulation by chronic stress is associated with increased α1c subunit expression in rat ventricular myocytes. Cell Stress Chaperon. 2009;14:33–41. doi: 10.1007/s12192-008-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]