Fig. 3.
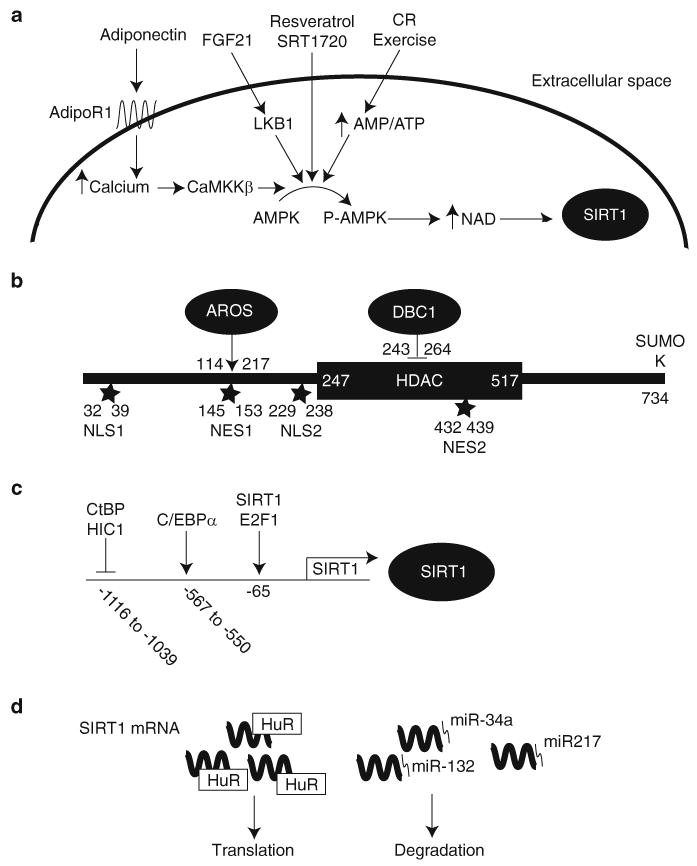
Regulation of SIRT1. SIRT1 activity is regulated by (A) NAD levels, (B) protein–protein interactions and posttranslational modifications, (C) factors that modulate its transcription, and (D) factors that modulate its translation. (A) SIRT1 activity is primarily regulated by NAD levels. NAD levels are regulated by AMPK-mediated up-regulation of NAMPT expression. AMPK can be activated by FGF21 and adiponectin. FGF21 induces LKB1, one of two major AMPK activators, to phosphorylate AMPK. Adiponectin induces calcium influx through its receptor adiponectin receptor 1 (adipoR1) and this calcium activates the other major AMPK kinase, calcium/calmodulin-dependent protein kinase kinase β (CaMKK β). (B) SIRT1 is activated by direct binding of AROS and sumoylation at Lysine734. Conversely, SIRT1 is inhibited by direct binding of DBC1. (C) p53 and HIC1 repress transcription of SIRT1 while C/EBPα and E2F1 enhance it. (D) Binding of HuR to SIRT1 mRNA increases its half-life. In contrast, binding of miR-34a, miR-132, or miR-217 results in translational repression of SIRT1 mRNA
