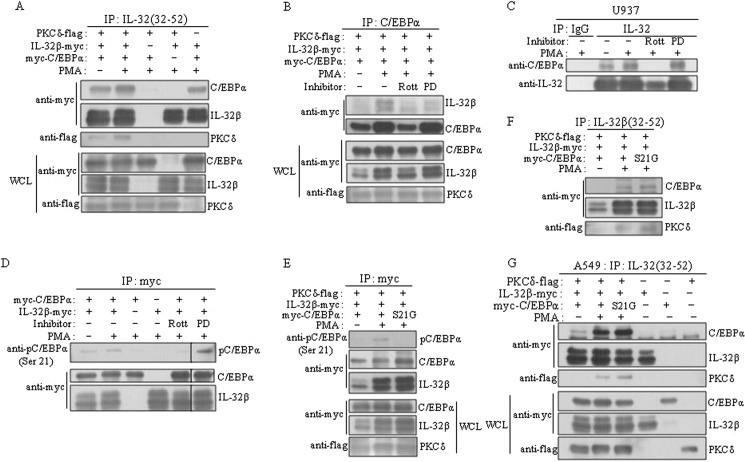
FIGURE 6.
IL-32β-mediated phosphorylation of C/EBPα Ser-21 by PKCδ. A and B, IL-32β was associated with C/EBPα and PKCδ, and then mediated the phosphorylation of C/EBPα serine 21 by PKCδ. HEK293 cells were co-transfected with 6×Myc-tagged IL-32β and C/EBPα and with 5×FLAG-tagged PKCδ. After overnight incubation, cells were treated with 10 μm rottlerin (Rott) or 10 μm PD98059 (PD) for 1 h for the inhibitor-treated samples. 20 nm PMA was treated for 90 min. Three micrograms of KU32-52 antibody (A) or C/EBPα antibody (B) was used. IP, immunoprecipitation; WCL, whole cell lysate. C, the expression levels of the transfected genes were determined by Western blotting with 30 μg of whole cell lysates (WCL). Immunoprecipitations (IP) were performed with U937 cells after 10 μm rottlerin or PD98059 treatment for 1 h and treatment with 20 nm PMA for a further 90 min. 3 μg of goat polyclonal IL-32 antibody was used. After co-transfection of HEK293 cells with 6×Myc-tagged IL-32β and C/EBPα, cells were treated with inhibitors and PMA in the same way as in A. D, cell lysates were immunoprecipitated with 1 μg of Myc tag antibody; the phosphorylated C/EBPα at serine 21 was detected using phospho-C/EBPα Ser-21-specific antibody. E and F, a mutant C/EBPα S21G was co-transfected with IL-32β and PKCδ into HEK293 cells, and then immunoprecipitations were performed with Myc tag antibody (E) or KU32-52 antibody (F). G, A549 lung carcinoma cells were co-transfected with a mutant C/EBPα S21G, IL-32β, and PKCδ. Each plasmid was also transfected as controls. Immunoprecipitation was carried out with Myc tag antibody. The expression levels of the introduced genes were determined by Western blotting with 30 μg of whole cell lysates (WCL).