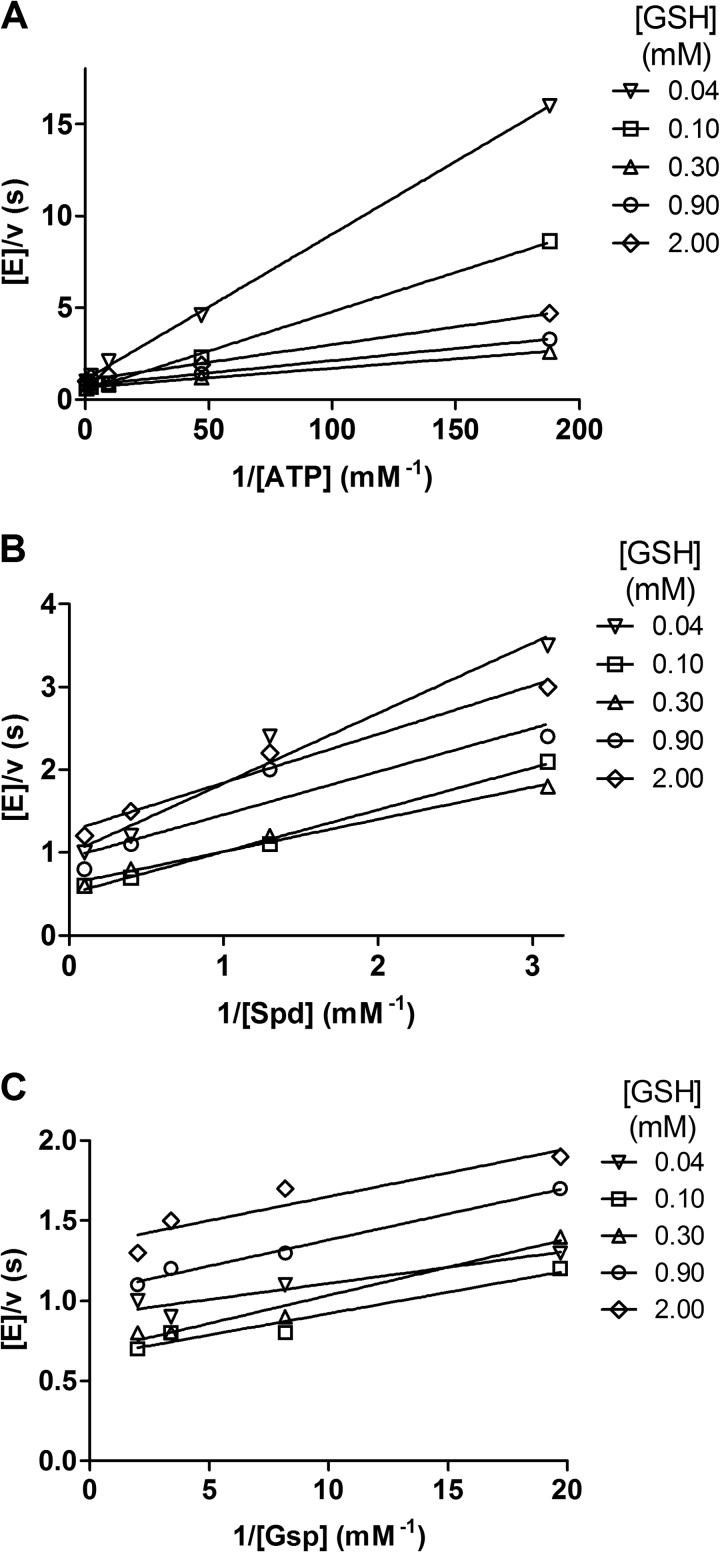
FIGURE 1.
Steady-state kinetic analysis of TryS. The activities were measured in the in vivo-like buffer system varying the concentration of two substrates while keeping one constant at saturating concentrations. Shown are double reciprocal plots of the reactions with fixed concentrations: 8 mm Spd (A), 2.3 mm ATP (B), and 2.3 mm ATP (C).