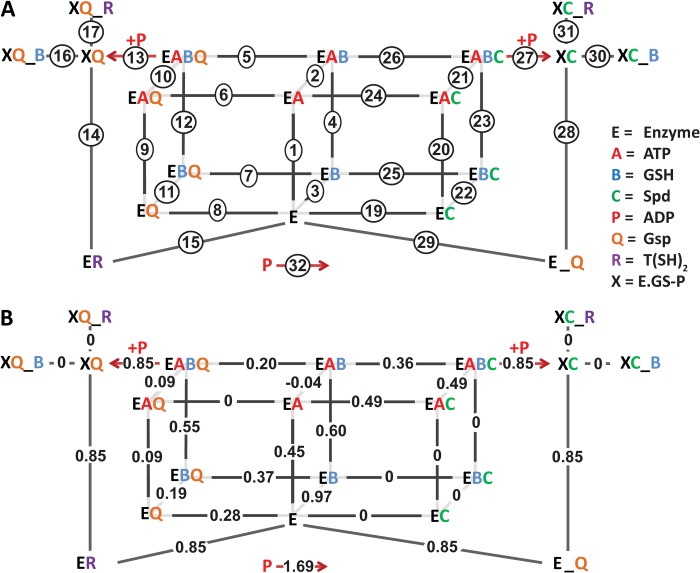
FIGURE 6.
Model of the ter-reactant mechanism of TryS catalysis. A, all reactions in the model consist of reversible mass action kinetics, except for those that generate ADP (reactions 13 and 27). The model includes a “coupling reaction” (reaction 32) that consumes the ADP formed in one (on Gsp) or two (on Spd) reactions. Compounds connected to the enzyme complex with an underscore (_) represent a complex that cannot proceed in the catalytic cycle, e.g. Gsp that is formed by the enzyme as product (E_Q) is in a wrong orientation in the substrate cavity for the second reaction. The activated enzyme complexes (XC or XQ) are the species formed after ATP and GSH reacted to generate a phosphorylated GSH intermediate and ADP. B, the numbers correspond to the steady-state flux values (μm/s) obtained during a simulation using 1 μm TryS and physiological substrate concentrations (2.3 mm ATP, 0.3 mm GSH, and 8 mm Spd). Positive values represent fluxes in the direction of product formation.