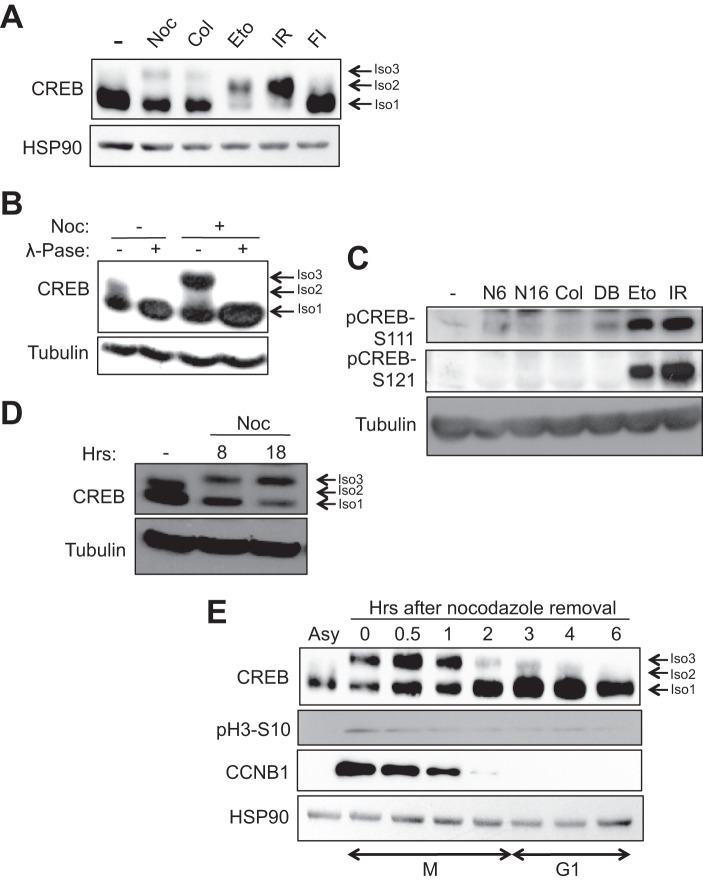
FIGURE 1.
DNA-damaging agents and anti-mitotic drugs elicit distinct changes in CREB electrophoretic mobility. A and B, HeLa cells were treated with DMSO, nocodazole (Noc), or colcemid (Col) for 16 h, etoposide (Eto) for 6 h, irradiation (IR) for 2 h, or forskolin and isobutylmethylxanthine (FI) for 90 min. Cell extracts were immunoblotted with α-CREB or α-HSP90. Isoform 1 (Iso1, bottom arrow) denotes unphosphorylated CREB, isoform 2 (Iso2, middle arrow) denotes CREB phosphorylated on the ATM/CK cluster (Ser-108, Ser-111, Ser-114, Ser-117, and Ser-121) (24, 25), and isoform 3 (Iso3, top arrow) denotes the CREB phospho-species selectively induced by microtubule poisons. B, cells were treated with DMSO or nocodazole for 8 h. Afterward, 30 μg of cell extracts were mock or λ-phosphatase (λ-Pase)-treated for 20 min at 37 °C. C, nocodazole does not induce CREB phosphorylation on DNA damage-inducible sites. HeLa cells were treated with DMSO, nocodazole for 6 h (N6) or 16 h (N16), colcemid for 16 h, dibutyryl cAMP (DB) (20 μm) for 90 min, etoposide for 6 h, or exposed to 10 Gy of γ-irradiation and harvested 2 h later. Cell extracts were run on a SDS-polyacrylamide gel and immunoblotted with α-pCREB-Ser-108/Ser-111/Ser-114 (pCREB-S111), α-pCREB-Ser-121, or α-β-tubulin. D, cells were treated with nocodazole for the indicated times. Cell extracts were immunoblotted with α-CREB or α-β-tubulin. E, kinetics of CREB phosphorylation during mitotic arrest and release. Cells were arrested in early mitosis by treatment with nocodazole for 16 h. Mitotic cells were isolated by mechanical shake-off and released into fresh medium. Cells were collected at the indicated times following nocodazole release. Cell extracts were immunoblotted with α-CREB, α-pH3-Ser-10, α-CCNB1, or α-HSP90.