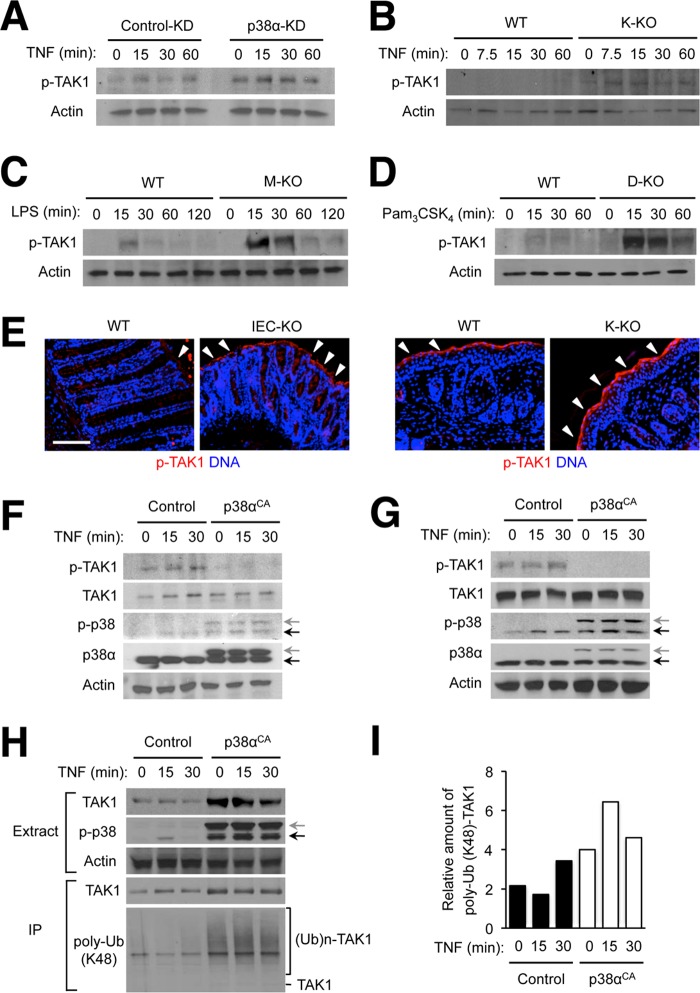
FIGURE 6.
Ablation of p38α expression results in TAK1 hyperactivation in various cell types. A–D, MODE-K cells (A), keratinocytes (B), bone marrow-derived macrophages (C), and lymph node dendritic cells (D) were prepared, treated, and analyzed by immunoblotting as described in Fig. 1. E, colon tissue sections from DSS-treated WT and IEC-KO mice were prepared as described in Fig. 5 (left). Skin tissue sections from TPA-treated WT and K-KO mice were prepared as described in Fig. 3 (right). Both types of tissue sections were analyzed by immunostaining with p-TAK1-specific antibodies. Arrowheads indicate areas where specific immunostaining is detected. Scale bar, 100 μm. F and G, MODE-K cells (F) and immortalized mouse embryonic fibroblasts (G) were transfected with control and constitutively active p38α (p38αCA)-expressing plasmid vectors, treated with TNF 24 h after transfection and analyzed as described in Fig. 1. Anti-p-p38 antibody detects both transfected (gray arrow) and endogenous p38 (black arrow). H and I, 293T cells were transfected and treated with TNF as described in F. A plasmid vector expressing HA epitope-tagged TAK1 was included in all transfection samples. Whole cell lysates (Extracts) were further subjected to immunoprecipitation with anti-HA antibody. Extracts and immunoprecipitated samples (IP) were analyzed by immunoblotting (H). The ratio of ubiquitinated and total HA-TAK1 amount in the immunoprecipitated proteins was determined by densitometry (I). (Ub)n-TAK1, polyubiquitinated (poly-Ub) TAK1.