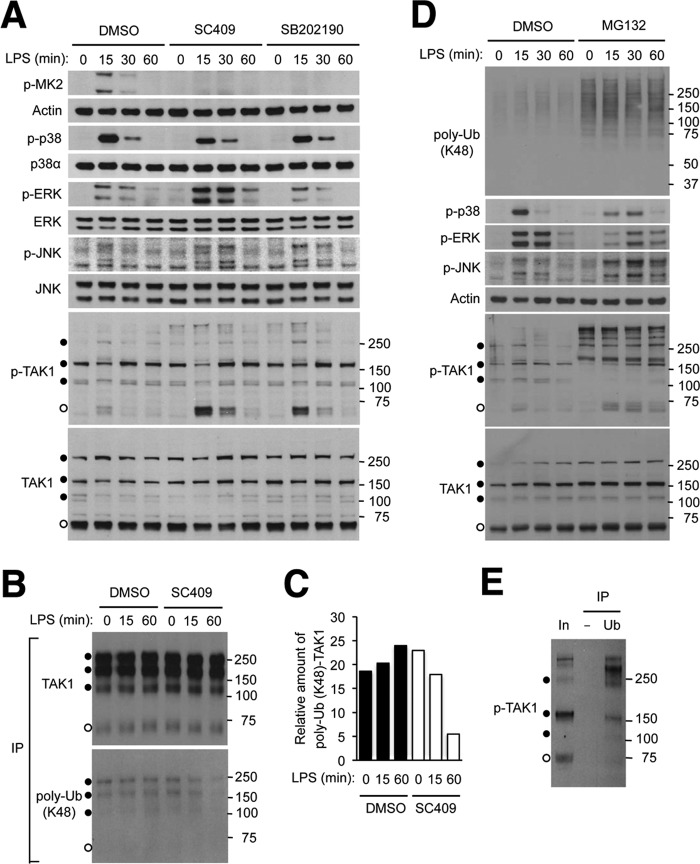
FIGURE 7.
Inhibition of p38α kinase activity results in TAK1 hyperactivation via affecting TAK1 ubiquitination and turnover. A, RAW264.7 mouse macrophage cells were preincubated with dimethyl sulfoxide (DMSO; 0.1%), SC409 (10 μm), and SB202190 (10 μm) before treatment with LPS (100 ng/ml). Whole cell lysates were prepared after the indicated durations of stimulation and analyzed by immunoblotting with antibodies against the proteins indicated on the left. Anti-p-TAK1 antibody and anti-TAK1 antibody detect proteins of higher apparent molecular weights (filled circles) in addition to those of the expected size (open circles). Numbers on the right indicate corresponding molecular weight marker positions. B and C, RAW264.7 cells were transfected with an HA-TAK1-expressing plasmid vector and treated with dimethyl sulfoxide, SC409, and LPS as described in A. Whole cell lysates were prepared and subjected to immunoprecipitation with anti-HA antibody and analyzed by immunoblotting (B). The ratio of ubiquitinated and total HA-TAK1 amount in the immunoprecipitated proteins was determined by densitometry (C). D and E, RAW264.7 cells were preincubated with dimethyl sulfoxide and MG132 (20 μm) before treatment with LPS. Whole cell lysates were prepared and analyzed by immunoblotting (D) as described in A. Whole cell lysate from cells preincubated with MG132 and treated with LPS for 60 min (corresponding to the eighth lane in D; In, Input) was subjected to immunoprecipitation with control (−) and anti-ubiquitin (Ub) antibody and analyzed by immunoblotting (E). poly-Ub, polyubiquitin.