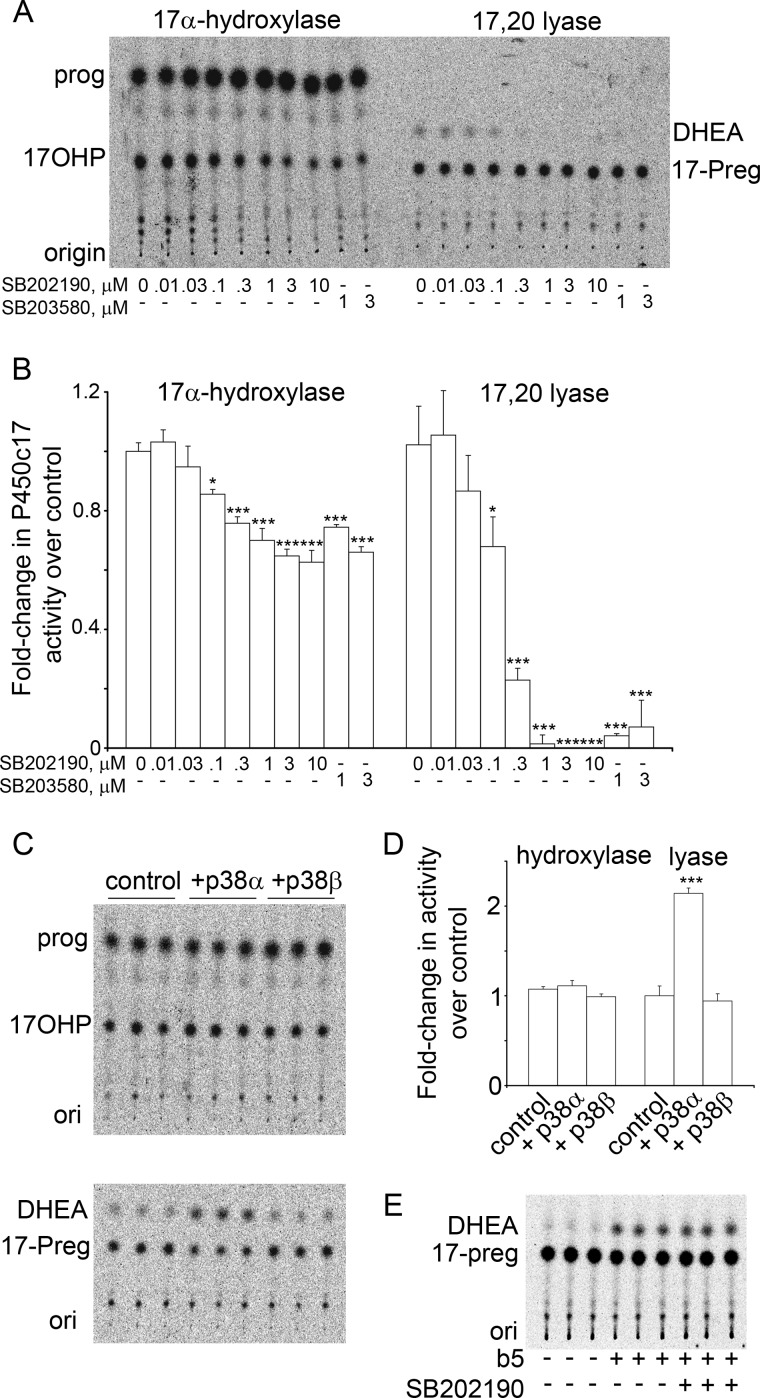
FIGURE 2.
p38α increases the 17,20 lyase activity of P450c17. A, TLC of one inhibitor experiment. Serum-starved NCI-H295A cells were treated for 4 h with the indicated doses (μm) of SB202190 and SB203580 and incubated with 1 μm [14C]progesterone (prog) (1 h) or 1 μm 17-OH-[3H]Preg (17-Preg) (1 h), and extracted media were analyzed by TLC. Both drugs inhibit 17,20 lyase activity (DHEA production) at 1 μm. B, pooled phosphorimaging data of three experiments done as in A. Data are compared with untreated control. Error bars represent S.E.; *, p < 0.05; ***, p < 0.001. C, TLC of steroids from COS-1 cells co-transfected with expression vectors for P450c17 and p38α or p38β. 17α-Hydroxylase activity (upper panel) was unchanged; p38α, but not p38β, increased 17,20 lyase activity 2.2-fold (lower panel). D, pooled data from three experiments, performed as in panel C, each performed in triplicate. (Error bars represent S.E.; ***, p < 0.001). E, SB202190 is not an enzymatic inhibitor of P450c17. Recombinant human P450c17 was incubated with cytochrome b5 to confer 17,20 lyase activity; the molar ratio of cytochrome b5:P450c17:POR was 1:1:2. The reaction was initiated with 1 mm NADPH. The presence of 10 μm SB202190, which ablated detectable 17,20 lyase activity in the cell system of A and B, had no effect on the 17,20 lyase activity of purified P450c17. ori, origin; 17OHP, 17-OH-progesterone; 17-Preg, 17-OH-pregnenolone.