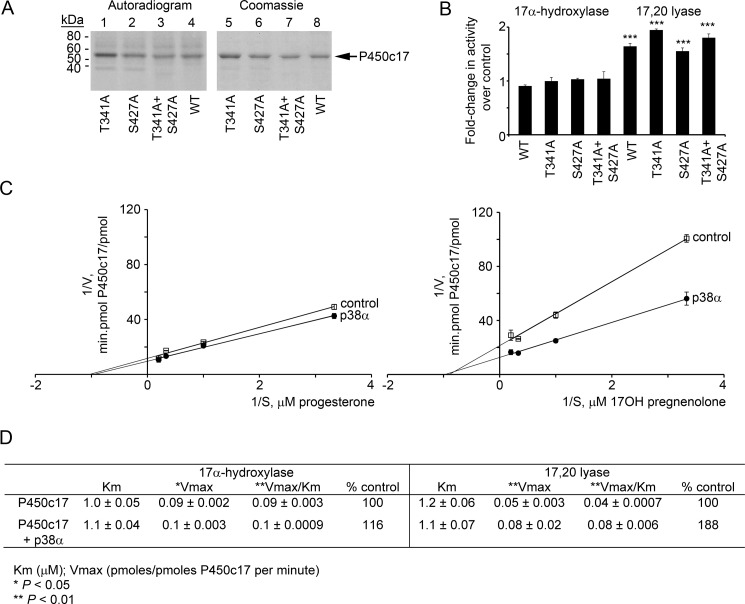
FIGURE 4.
Phosphorylation of P450c17 does not occur at canonical (Ser/Thr)-Pro sites. A, the bacterially expressed human P450c17 mutants T341A, S427A, and T341A/S427A (lanes 1–3) and wild-type human P450c17 (lane 4) were phosphorylated in vitro with p38α, separated by 10% PAGE-SDS, and imaged by autoradiography (left) and by Coomassie staining (right). B, mutation of the canonical p38α sites in P450c17 does not affect the action of p38α. Wild-type (WT), T341A, S427A, and T341A/S427A P450c17 were expressed in COS-1 cells with co-transfection of a vector expressing p38α, and their 17α-hydroxylase and 17,20 lyase activities were compared with the activities of the P450c17 constructs without co-transfected p38α. Radiolabeled steroid products of the assays were separated by TLC, autoradiographed, and quantitated using Scion Image software (Frederick, MD). The 17α-hydroxylase activities of the phospho- and dephospho-P450c17 did not differ (left), but all four phosphorylated forms of P450c17 had significantly increased 17,20 lyase activity as assessed by Vmax/Km (p < 0.001 for all four bars). C, Lineweaver-Burk analysis of enzyme kinetics. Data are from three independent experiments, each performed in triplicate, and are shown ±S.E. (n = 3). D, kinetic data calculated from C. *, p < 0.05; **, p < 0.01; ***, p < 0.001.