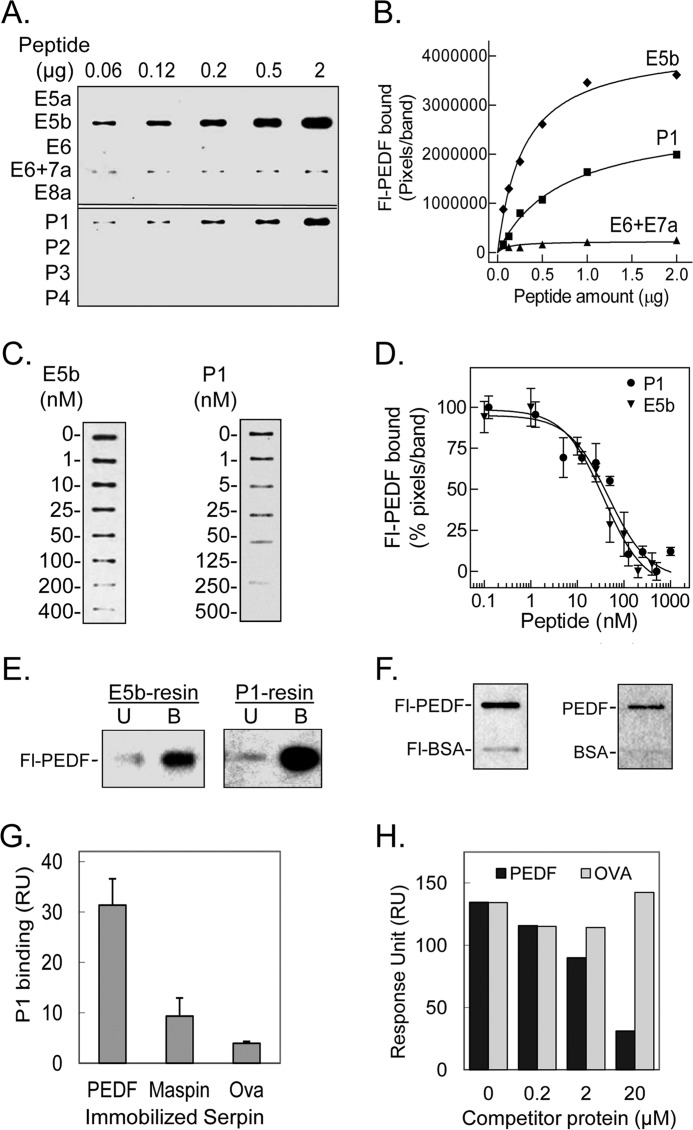
FIGURE 3.
Binding of PEDF to peptides derived from L4 of PEDF-R. A, ligand blot of PEDF-R-derived peptides. Increasing amounts of the indicated peptide were immobilized per slot on a nitrocellulose membrane. After blocking, the ligand Fl-PEDF at 1 nm in 0.1% BSA TBS-T was added to the membrane and incubated for 1.5 h at room temperature. Bound Fl-PEDF was detected by Western blotting versus anti-fluorescein. B, quantification of PEDF bound to PEDF-R-derived peptides. The intensity of the bands in A was densitometrically quantified using UN-SCAN-IT software, and pixels per band were plotted versus the amount of peptide, as indicated. C, competition of Fl-PEDF binding to immobilized peptides with E5b and P1 in solution. E5b or P1 (1 μg) were immobilized on to the nitrocellulose membrane in triplicates per condition. After blocking, the membrane was incubated with a mixture of 1 nm Fl-PEDF and increasing concentrations of E5b or P1 (as indicated) in 0.1% BSA-TBS-T. Bound Fl-PEDF was either detected directly using the fluorescence imager or as described above. One set of slots for each peptide is shown. D, the bands in C were quantified by densitometry. The intensities of the bands in percentage of pixels per band relative to without peptide in solution were determined and plotted as a function of the concentration of peptide in solution. Each point corresponds to the average of triplicates ± S.E. (error bars) per concentration. The data were normalized and analyzed using GraphPad Prism software version 5 with an equation for binding, competitive with one site, fit log IC50. The estimated IC50 values for E5b and P1 were 37.5 ± 1.4 and 47.2 ± 1.3 nm, respectively. E, PEDF and Fl-PEDF (300 ng) were subjected to E5b peptide or P1 peptide affinity chromatography in Tris-buffered saline containing 0.05% Tween 20 (TBS-T). Flow-through containing the unbound material was collected. After extensive washes with binding buffer, bound protein was eluted with SDS-PAGE sample buffer. Western blots of unbound (U) and bound (B) protein versus anti-PEDF are shown. F, PEDF and BSA conjugated and non-conjugated with fluorescein were immobilized on nitrocellulose membranes. E5b in solution at 200 nm was used as the ligand for the blot. Ligand blots immunoreacted with anti-PEDF-R are shown. G, comparison of P1 binding response to PEDF and other serpins. Serpins were immobilized on a sensor chip, and the analyte (P1 at 2 μm) was injected over the surface. Responses to the same amount of P1 injected on immobilized maspin, ovalbumin (Ova), and PEDF surfaces are shown. H, competition of P1 binding to PEDF immobilized on a CM5 sensor chip with PEDF or ovalbumin in solution. Mixtures of P1 (2 μm) with the indicated concentrations of serpins were injected over the surface as analytes. Relative response as a function of time is plotted.