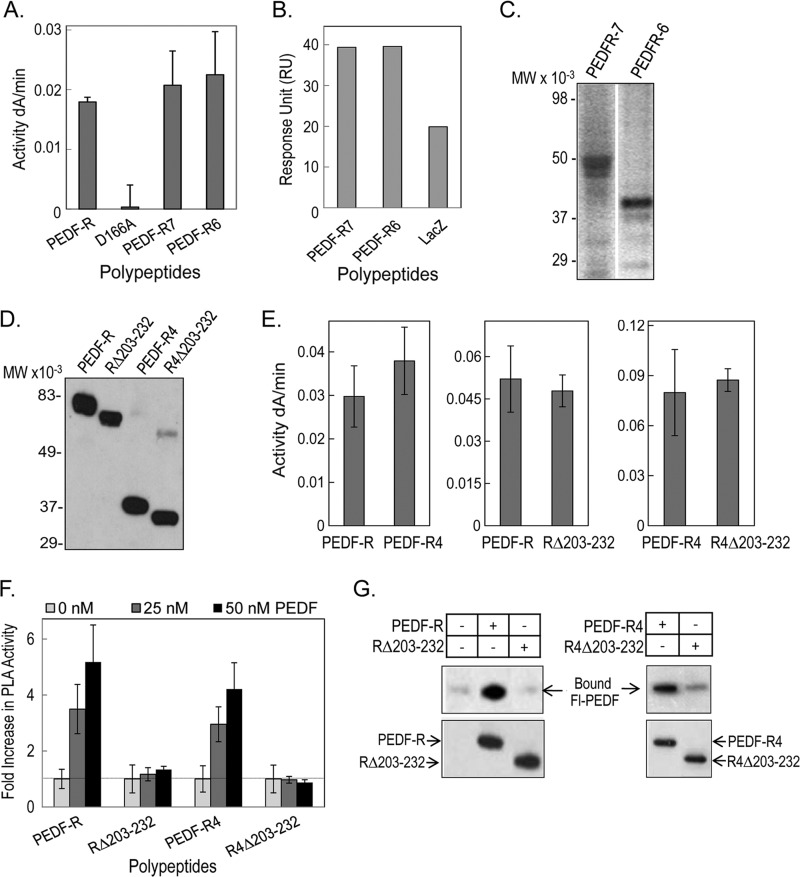
FIGURE 4.
Phospholipase activity and PEDF binding of truncated PEDF-R and PEDF-R lacking the ligand binding region (His203–Leu232). A, PLA2 activity of recombinant PEDF-R variants. PLA2 activity assays were performed with 1,2-dilinoleoyl-phosphatidylcholine as the substrate, 20 μl of the purified His6/Xpress-tagged PEDF-R variant polypeptides (∼0.6 pmol/reaction), and lipoxygenase as the coupling enzyme in PLA2 reaction buffer (3 mm deoxycholate, 5 mm Tris-Cl, pH 7.5). The appearance of linoleoyl hydroperoxide, the coupled reaction product, was measured spectrophotometrically by increasing absorbance at 234 nm/min for 10 min at room temperature (n = 4). B, comparison of PEDF-R binding response to PEDF. Maximum response units after injection of PEDF-R polypeptides are plotted. PEDF was immobilized on a CM5 sensor chip, and PEDF-R polypeptides were the analytes injected over the surface. C, Western blot of purified protein fractions of PEDF-R7 and PEDF-R6 versus anti-Xpress. A total of 20 μl of each fraction were resolved by SDS-PAGE. D, Western blot of purified recombinant His6/Xpress-tagged PEDF-R, PEDF-RΔ203–232 (RΔ203–232), PEDF-R4, and PEDF-R4Δ203–232 (R4Δ203–232) proteins versus anti-Xpress. A total of 20 μl of each fraction were resolved by SDS-PAGE. E, PLA2 activity assays of recombinant PEDF-R variants were performed as in A. F, effect of PEDF additions on the PLA2 activity of PEDF-R polypeptides. Equivalent amounts of affinity-purified PEDF-R and variant polypeptides (∼0.6 pmol/reaction) were preincubated with increasing amounts of PEDF in PLA2 reaction buffer for 10 min on ice. PLA2 activity was measured as above and represented as -fold change over basal activity without PEDF. Shown is the average -fold change from two independent experiments (n = 4). G, binding assays by His tag pull-down. Soluble fractions of cell-free expression reactions containing His6/Xpress-tagged PEDF-R, RΔ203–232, PEDF-R4, and R4Δ203–232 (∼700 ng each) were mixed with Fl-PEDF protein (250 ng) in PLA2 reaction buffer and incubated for 2 h at 4 °C with gentle rotation. This was followed by the addition of Ni-NTA resin beads (25 μl), and the suspension was incubated for 1 h at 4 °C with gentle rotation. Bound PEDF (pull-down) was extracted with SDS-sample buffer and analyzed by Western blot versus anti-PEDF. Error bars, ±S.D.