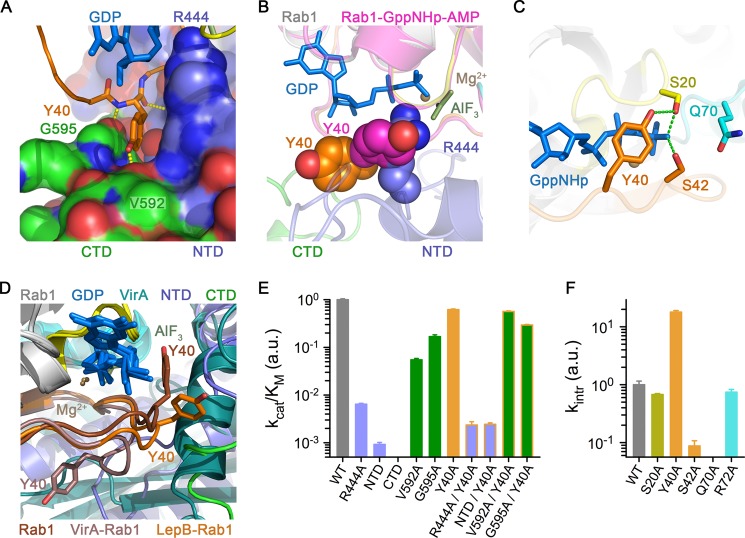
FIGURE 6.
Role of the tandem domain architecture and clamp interaction. A, shown are polar and non-polar interactions between Tyr-40 in the switch I region of Rab1 and the interface of the N-terminal and C-terminal domains of the LepB GAP core. CTD, C-terminal domain; NTD, N-terminal domain. B, shown is a comparison of Rab1 from the LepB complex with AMPylated Rab1-GTP (PDB ID 3NKV) after superposition. C, shown are polar interactions involving the Ser-20, Tyr-40, and Ser-42 in the closed conformation of AMPylated Rab1-GTP. D, shown is a comparison of Tyr-40 conformations in AMPylated Rab1-GTP alone and in the complexes with LepB and VirA (PDB ID 4FMB) after superposition of the Rab1 molecules. E, shown is catalytic efficiency of LepB and/or Rab1 mutations involving residues or domains implicated in the clamp interaction with Tyr-40. F, shown are rate constants for intrinsic GTP hydrolysis by Rab1 mutants.