FIGURE 7.
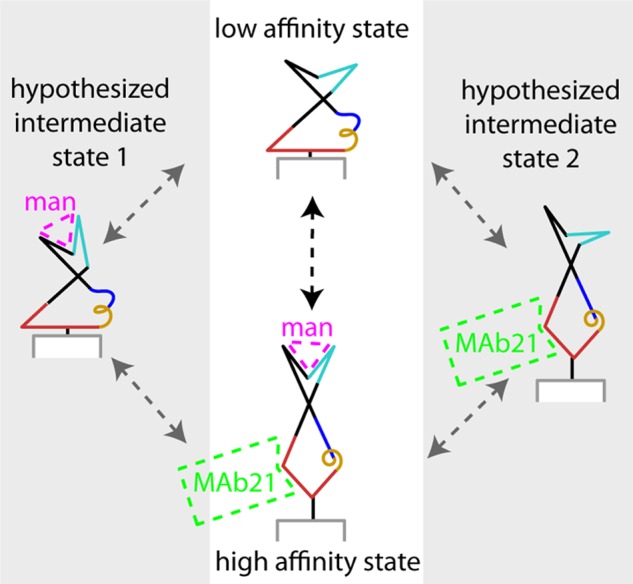
Schematic of allosteric coupling. The stable low and high affinity conformations are shown in the central white panel. The different conformations of the clamp loop and pocket zipper (cyan), interdomain loops (red) α-helix (gold) and β-bulge (blue) illustrate the conclusions that all are involved in the allosteric mechanism. The gray side panels illustrate the hypothesis that weak coupling of the clamp loop and pocket zipper to the interdomain loops, α-helix and β-bulge, could result in intermediate states in which one of these regions is in the high affinity conformation and the other low. If so, the allosteric transition would occur through one of these intermediates (gray arrows) rather than in a single concerted reaction (black arrow). When the clamp loop and pocket zipper (cyan) are in the high affinity conformation, mannose is shown binding in magenta. When the interdomain loops, α-helix and β-bulge (red, gold, and blue, respectively) are in the high affinity conformation, mAb21 is shown binding in green. Part of the pilin domain is shown in gray and white.
