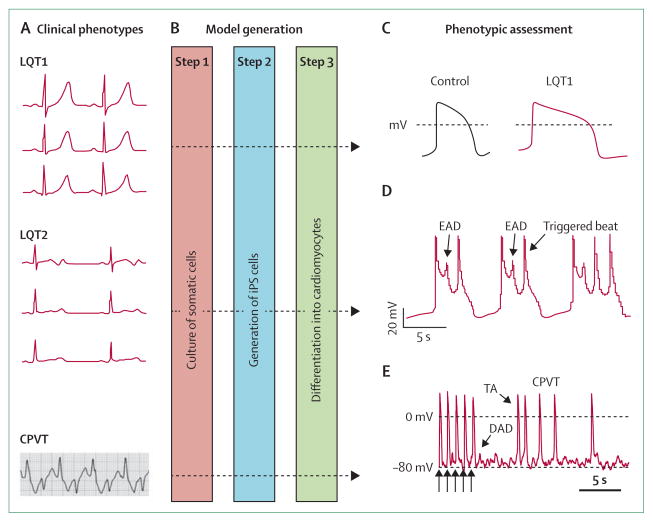
Figure 5. Induced pluripotent stem (iPS)-cell models of ion-channel diseases.
(A) iPS-cell models of long QT syndromes and catecholaminergic polymorphic ventricular tachycardia (CPVT) have been established (figure shows representative electrocardiograms of each).20,43,44 (B) Steps 1–2: fibroblasts and other somatic cells can be reprogrammed to become iPS cells after transduction with retroviral vectors that express four transcription factors (POU5F1 [also known as OCT4], SOX2, KLF4, and MYC).8 Step 3: directed differentiation into cardiomyocytes provides cellular models for physiological assessment. Cardiomyocytes from patients with long QT syndrome 1 (LQT1) that harbour an Arg190Gln variant in KCNQ3 recapitulate features of clinical phenotypes (C), with prolonged action potentials, defective potassium-channel characteristics, and inducible arrhythmias attenuated by β-adrenergic blockade.43 Similar characteristics are seen with cells from LQT2 patients—the Ala614Val variant in KCNH3 (D) shows early afterdepolarisations (EADs) and triggered activity.44 Typical action potentials from a ventricular myocyte obtained from a patient with CPVT that carried a Ser406Leu variant in RYR3 (E) show 5 paced beats (arrows) followed by delayed afterdepolarisations (DADs) and triggered activity (TA).20 (C) is reproduced from reference 43, by permission of the Massachusetts Medical Society; (D) is reproduced from reference 44, by permission of Nature Publishing Group; (E) is reproduced from reference 20, by permission of John Wiley and Sons.