Abstract
Brassinosteroid (BR)-induced antioxidant defence has been shown to enhance stress tolerance. In this study, the role of the maize 65kDa microtubule-associated protein (MAP65), ZmMAP65-1a, in BR-induced antioxidant defence was investigated. Treatment with BR increased the expression of ZmMAP65-1a in maize (Zea mays) leaves and mesophyll protoplasts. Transient expression and RNA interference silencing of ZmMAP65-1a in mesophyll protoplasts further revealed that ZmMAP65-1a is required for the BR-induced increase in expression and activity of superoxide dismutase (SOD) and ascorbate peroxidase (APX). Both exogenous and BR-induced endogenous H2O2 increased the expression of ZmMAP65-1a. Conversely, transient expression of ZmMAP65-1a in maize mesophyll protoplasts enhanced BR-induced H2O2 accumulation, while transient silencing of ZmMAP65-1a blocked the BR-induced expression of NADPH oxidase genes and inhibited BR-induced H2O2 accumulation. Inhibiting the activity and gene expression of ZmMPK5 significantly prevented the BR-induced expression of ZmMAP65-1a. Likewise, transient expression of ZmMPK5 enhanced BR-induced activities of the antioxidant defence enzymes SOD and APX in a ZmMAP65- 1a-dependent manner. ZmMPK5 directly interacted with ZmMAP65-1a in vivo and phosphorylated ZmMAP65-1a in vitro. These results suggest that BR-induced antioxidant defence in maize operates through the interaction of ZmMPK5 with ZmMAP65-1a. Furthermore, ZmMAP65-1a functions in H2O2 self-propagation via regulation of the expression of NADPH oxidase genes in BR signalling.
Key words: Antioxidant defence, brassinosteroid, H2O2, NADPH oxidase, ZmMAP65-1a, ZmMPK5.
Introduction
Brassinosteroids (BRs) are a class of steroid hormones controlling various growth and developmental processes in plants, including cell division and expansion, photomorphogenesis, xylem differentiation, floral development, and seed germination (Clouse and Sasse, 1998; Bishop and Koncz, 2002; Bajguz, 2007; Choudhary et al., 2012). In addition, BRs have also been demonstrated to regulate biotic and abiotic stress responses in plants (Kagale et al., 2007; Divi and Krishna, 2009; Xia et al., 2009; Zhang et al., 2011; Wang, 2012). Several studies have shown that exogenously applied BR enhances the tolerance to oxidative, Cu and Cr, and cold stress, and is accompanied by the accumulation of H2O2 and the enhancement of antioxidants enzymes, such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (Xia et al., 2009; Choudhary et al., 2010, 2011, 2012; Zhang et al., 2010; Cui et al., 2011), which scavenge excessive reactive oxygen species (ROS) in distinct organelles (Foyer and Noctor, 2005; Tan et al., 2011). However, it remains largely unknown how BRs induce ROS production and upregulate antioxidant defence.
The plant cytoskeleton can be readily remodelled in response to a variety of intracellular and external stimuli. As an important component of the cytoskeleton, microtubules (MTs) have been well documented to be essential for intra- and extracellular signalling, and the regulation of the dynamic instability of MTs plays a critical role in MT function, such as in the plant’s ability to withstand salt and osmotic stress (Mathur and Chua, 2000; Nogales, 2000; Lü et al., 2007; Sedbrook and Kaloriti, 2008; Wang et al., 2011a). MT dynamic instability is precisely regulated by microtubule-associated proteins (MAPs) (Desai and Mitchison, 1997).
MAP65 is one of the most abundant plant MAPs (Jiang and Sonobe, 1993). The first members of the MAP65 family of proteins were isolated from tobacco BY2 cells as a group of 60–65kDa proteins that co-purified with MTs (Jiang and Sonobe, 1993). Subsequently, MAP65 proteins were identified respectively in other plants. Arabidopsis has nine MAP65 proteins with predicted molecular masses between 54 and 80kDa (Hussey et al., 2002). The rice genome encodes 11 members of the MAP65 family (Guo et al., 2009). These proteins have evolved to take on distinct tasks required for multifaceted cellular activities. MAP65 has been shown to be responsible for the bundling of cortical MTs during secondary cell-wall formation in xylogenesis and during the expansion of primary cell walls (Mao et al., 2006). In addition, many studies have shown that MAP65 proteins not only play critical roles for cell division and elongation, root growth, and leaf senescence (Keech et al., 2010; Lucas et al., 2011; Soares et al., 2011; Dhonukshe et al., 2012), but are also required for stabilizing MTs during low temperature and NaCl stress (Smertenko et al., 2004; Mao et al., 2005). In addition, MAP65 is essential for giant-cell development during root knot nematode infection (Caillaud et al., 2008). These findings show that MAP65 proteins are required both for developmental processes and for responses to biotic and abiotic stress. However, little is known about how MAP65 proteins function in response to stresses.
Recent studies have reported that cytoskeleton (i.e. actin filament) reconfiguration is sufficient to activate BR signalling (Lanza et al., 2012), BR treatment induced H2O2 production and enhanced the activities of antioxidant enzymes (Zhang et al., 2010), and disturbance of ROS homeostasis resulted in MT and atypical tubulin formation and aggregation of MAP65 (Livanos et al., 2012). These observations suggest that there might be a link between MAP65 and BR signalling. Here, this hypothesis was tested and our experimental results showed that maize MAP65, ZmMAP65-1a, interacts with ZmMPK5 and is required for BR-induced antioxidant defence.
Materials and methods
Plant material and treatments
Seeds of maize (Zea mays L. cv. Nongda 108; from Nanjing Agricultural University, China) were sown in trays of sand in a growth chamber at a temperature of 22–28 °C, photosynthetic active radiation of 200 μmol m−2 s−1, and a photoperiod of 14/10h (day/night), and were watered daily. When the second leaves were fully expanded, they were collected and used for investigations.
The plants were excised at the base of the stem and placed in distilled water for 1h to eliminate wound stress. After treatment, the cut ends of the stems were placed in beakers wrapped with aluminum foil containing 10nM BR or 10mM H2O2 solution for various times at 25 °C, with a continuous light intensity of 200 μmol m−2 s−1. In order to study the effects of various inhibitors or scavengers, the detached plants were pre-treated with 100 μM 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD98059), 10 μM 1,4-diamino-2,3- dicyano-1,4-bis(o-aminophenylmercapto) butadiene (U0126), 5mM dimethylthiourea (DMTU), 100 μM diphenylene iodonium (DPI) or 200U CAT for 4h prior to treatment with 10nM BR as described above. Detached plants were treated with distilled water under the same conditions for the whole period and served as controls for the above. After treatment of the detached plants, the second leaves were sampled and immediately frozen in liquid N2.
Isolation of total RNA and real-time quantitative reverse transcriptase-PCR (qRT-PCR) expression analysis
Total RNA was isolated from leaves or protoplasts using an RNAiso Plus kit (TaKaRa, Dalian, China) according to the instructions supplied by the manufacturer. DNase treatment was included in the isolation step using the RNase-free DNase (TaKaRa). Approximately 2 μg of total RNA was reverse transcribed using an oligo(dT)16 primer and Moloney murine leukemia virus reverse transcriptase (TaKaRa). Transcript levels of several genes were measured by qRT-PCR using a DNA Engine Opticon 2 real-time PCR detection system (Bio-Rad, USA) with SYBR® Premix Ex Taq TM (TaKaRa) according to the manufacturer’s instructions. The cDNA was amplified by PCR using the following primers: ZmSOD4 (GenBank accession no. NM_001112234), forward 5′-TGGAGC ACCAGAAGATGA-3′ and reverse 5′-CTCGTGTCCAC CCTTTCC-3′; ZmAPX2 (EU969033), forward 5′-TGAGCGACC AGGACATTG-3′ and reverse 5′-GAGGGCTTTGTCACTT GGT-3′; ZmMAP65-1a (EU972149), forward 5′-AAGAGGA AAGTTGACC-3′ and reverse 5′-TGCTTGATTGTCCCTGT-3′; ZmMPK5 (AB016802), forward 5′-TCTGCTCGGCGGTCAACT-3′ and reverse 5′-AAGGCGTTGGCGATCTTCTT-3′; ZmrbohA (DQ855284), forward 5′-CACACGTGACCTGCGACTTC-3′ and reverse 5′-CCCCAAGGTGGCCATGA-3′; ZmrbohB (EU807966), forward 5′-GGCCAGTACTTCGGTGAAACA-3′ and reverse 5′-ATTACACCAGTGATGCCTTCCA-3′; ZmrbohC (DQ897930), forward 5′-TTCTCTTGCCTGTATGCCGC-3′ and reverse 5′-CTTTCGTATTCCGCAGCCA-3′; ZmrbohD (EF364442), forward 5′-CCGGCTGCAGACGTTCTT-3′ and reverse 5′-CCTGATCCGTGATCTTCGAAA-3′; ZmACTIN (EU952376), forward 5′-GCCATCCATGATCGGTATGG-3′ and reverse 5′-GTCGCACTTCATGATGGAGTTG-3′. To standardize the results, amplification of ZmACTIN was determined and used as the internal standard. The data were normalized to amplification of the maize ZmACTIN gene. For each sample, the mean value from three qRT-PCRs was adapted to calculate the expression abundance, and the mean values were then plotted with their standard error (SE).
Vector construction and in vitro transcription of ZmMAP65-1a dsRNA
The full-length cDNA fragment was amplified with the addition of a KpnI site and then inserted in frame with yellow fluorescent protein (YFP) into the pXZP008 vector driven by the cauliflower mosaic virus 35S promoter. The primers used for the PCR amplification were: 5′-GGTACCGATGGCCGGTGACATTACATGCGG-3′ and 5′-GGTACCCGTGGTGTGCTCGGAACCGGATC-3′.
DNA templates were produced by PCR using primers containing the T7 promoter sequence (5′-TAATACGACTCACTATAGGC-3′) on both 5′ and 3′ ends. The primers used to amplify DNA of ZmMAP65-1a were: 5′-TAATACGACTCACTATAGGCGCGTCT CAAACGGCACT-3′ and 5′-TAATACGACTCACTATAGGC TGTCTTTCTTGCTATCCTTCC-3′. The PCR conditions were as follows: denaturing step at 94 °C for 5min, followed by 35 cycles of 94 °C for 15 s, 67 °C for 15 s, and 72 °C for 15 s, with a final extension at 72 °C for 10min. After PCR product clean-up, the DNA templates were used for in vitro synthesis of dsRNA using a Ribomax Express kit (Promega, USA). The dsRNA was purified by phenol/chloroform/isopropanol extraction, dissolved in RNase-free water, and quantified by UV spectrophotometry.
Protoplast preparation and transfection with DNA constructs or dsRNAs
Maize plants were grown at 25 °C under dark conditions. When the second leaves were fully expanded, protoplasts were isolated from leaves used for transfection with DNA constructs or dsRNAs based on the protocol for maize mesophyll protoplasts provided online by J. Sheen’s laboratory (http://genetics.mgh.harvard.edu/sheenweb) with minor modifications. For transfection, 1ml of maize protoplasts (usually 5×105 cells ml−1) were transfected with 100 μg of 35S–ZmMAP65-1a–YFP fusion construct (using the pXZP008 vector as a control), 35S–ZmMAP65-1a–mCherry (using the pXZP008 vector as a control), or 150 μg of dsRNAs (using H2O as a control) using a polyethylene glycol/calcium-mediated method. The transfected protoplasts were incubated in incubation solution overnight in the dark at 25 °C, and the protoplasts were then collected and used for further analysis.
Antioxidant enzyme assays
Protoplasts were homogenized in 0.7ml of 50mM potassium phosphate buffer (pH 7.0) containing 1mM EDTA and 1% polyvinylpyrrolidone, with the addition of 1mM ascorbate in the case of the APX assay. The homogenate was centrifuged at 15 000g for 20min at 4 °C and the supernatant was immediately used for the following antioxidant enzyme assays. The total activities of antioxidant enzymes were determined as previously described (Zhang et al., 2006). Total SOD activity was assayed by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium. One unit of SOD activity was defined as the amount of enzyme that was required to cause 50% inhibition of the reduction of nitro blue tetrazolium, as monitored at 560nm. Total APX activity was measured by monitoring the decrease in absorbance at 290nm as ascorbate was oxidized.
H2O2 detection by confocal laser-scanning microscopy
H2O2 production in protoplasts was monitored using the H2O2-sensitive fluorescent probe H2DCF-DA (Molecular Probes, Leiden, The Netherlands) using the method described by Bright et al. (2006). Images acquired were analysed using Leica IMAGE software. Data are presented as mean fluorescence intensity.
Expression and purification of recombinant ZmMAP65-1a
Full-length ZmMAP65-1a was cloned into EcoRI/XhoI-digested pGEX4T-1 vector to generate a glutathione S-transferase (GST)–ZmMAP65-1a construct. Fusion proteins were expressed in Escherichia coli strain BL21(DE3) according to the manufacturer’s instructions. Protein expression was induced with isopropyl β-d-1-thiogalactopyranoside for 4h in Luria–Bertani liquid medium. The bacteria were collected at 5000g for 15min, resuspended in PBS (pH 8.0), sonicated, and centrifuged at 12 000g for 10min. The resulting supernatant was used for protein purification with GST-affinity agarose (Genscript, Nanjing, China) according to the manufacturer’s instructions. Purified proteins were used for immunoblotting and an immunoprecipitation kinase activity assay.
Immunoblotting
Purified proteins were subjected to SDS-PAGE. Immunoblotting was performed as described by Ma et al. (2012). Anti-GST antibody (Abmart, Shanghai, China) was used to detect the GST–ZmMAP65-1a protein.
Antibody production and immunoprecipitation kinase activity assay
The peptide ZmMPK5-C (EEQMKDLIYQEALAFNPDYQ) corresponding to the C terminus of ZmMPK5 was synthesized as described by Berberich et al. (1999) and conjugated to keyhole limpet haemocyanin. ZmMPK5 polyclonal antibody was raised in rabbits and purified by affinity chromatography.
Protein was extracted from maize leaves as described previously (Zhang et al., 2006). Protein content was determined according to the method of Bradford (1976) with BSA as a standard. For the immunoprecipitation kinase assay, protein extract (200 μg) was incubated with anti-ZmMPK5 antibody (7.5 μg) in an immunoprecipitation buffer as described previously (Zhang et al., 2006). Kinase activity in the immunocomplex was determined by an in-gel kinase assay using GST–ZmMAP65-1a fusion protein as the substrate. The immunocomplex and GST–ZmMAP65-1a were incubated in reaction buffer (25mM Tris/HCl, pH 7.5, 5mM MgCl2, 1mM DTT, 1mM EGTA) with 200nM ATP and 1 μCi of [γ-32P]ATP (3000 Ci mmol−1) for 30min. An equal volume of SDS sample buffer was added to stop the reaction. The reaction mix was boiled for 5min and resolved by SDS-PAGE. Unincorporated [γ-32P]ATP was removed by washing with 5% trichloroacetic acid (w/v)/1% sodium pyrophosphate (w/v) at least three times. The gel was dried onto Whatman 3MM paper and exposed to Kodak XAR-5 film. Pre-stained size markers (Bio- Rad) were used to calculate the apparent molecular mass.
Bimolecular fluorescence complementation (BiFC) analysis of the interaction between ZmMPK5 and ZmMAP65-1a
Onion epidermal cells were co-transfected with the expression vectors YFPN–ZmMPK5 and YFPC–ZmMAP65-1a by DNA particle bombardment according to the manufacturer’s instructions (Biolistic PDS-1000/He Particle Delivery System; Bio-Rad). Co-expression of YFPN and YFPC, YFPN–ZmMPK5 and YFPC, and YFPN and YFPC–ZmMAP65-1a in onion epidermal cells were used as negative controls. YFP fluorescence was detected after 12–16h of transfection.
Results
BR upregulates the expression of ZmMAP65-1a
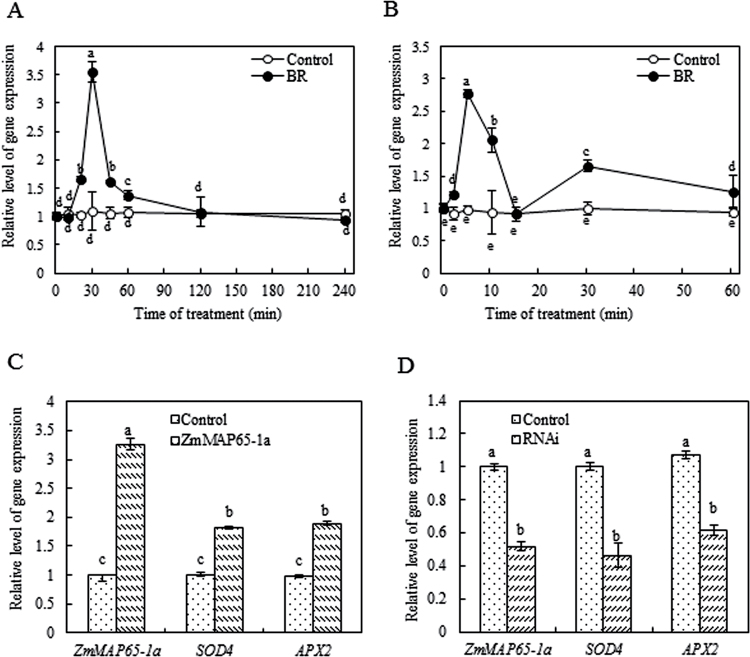
To investigate whether ZmMAP65-1a participates in BR signalling, total RNA was isolated from maize leaves or protoplasts treated with 10nM BR, and the expression of ZmMAP65-1a was analysed by real-time qRT-PCR analysis. As shown in Fig. 1A, 10 nM BR treatment induced a rapid increase in the expression of ZmMAP65-1a in maize leaves. The expression of ZmMPP65-1a was upregulated after 20min, peaked after 30min, and then decreased after 45min of BR treatment in the leaves of maize plants (Fig. 1A). In protoplasts, the expression of ZmMAP65-1a was even more rapidly upregulated by the treatment of 10nM BR (Fig. 1B).
Fig. 1.
BR induces the expression of ZmMAP65-1a, and ZmMAP65-1a is required for the expression of SOD4 and APX2 in maize. (A, B) Expression analysis of ZmMAP65-1a in maize leaves (A) or mesophyll protoplasts (B) exposed to BR treatment. The maize seedlings or protoplasts were treated with 10nM BR for various times as indicated. Seedlings treated with distilled water and protoplasts treated with culture medium under the same conditions served as controls. Relative expression level of ZmMAP65-1a was analysed by real-time qRT-PCR. (C) Expression analysis of ZmMAP65-1a, SOD4 and APX2 in protoplasts transiently expressing ZmMAP65-1a. Protoplasts isolated from maize leaves were transfected with constructs carrying 35S–ZmMAP65-1a–YFP (ZmMAP65-1a). Protoplasts were transfected with empty vector as a control. The relative expression levels of ZmMAP65-1a, SOD4 and APX2 were analysed by real-time qRT-PCR. (D) Expression analysis of ZmMAP65-1a, SOD4 and APX2 in protoplasts transiently silencing ZmMAP65-1a. Protoplasts were transfected with dsRNA against ZmMAP65-1a (RNAi) or distilled water as a control. The relative expression levels of ZmMAP65-1a, SOD4 and APX2 were analysed by real-time qRT-PCR. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P <0.05 according to Duncan’s multiple range test.
ZmMAP65-1a is required for BR-induced antioxidant defence
Previous studies have showed that BR can induce antioxidant defence to enhance stress tolerance (Xia et al., 2009; Zhang et al., 2010) and that MAP65 plays a role in the regulation of MTs against stresses (Livanos et al., 2012; Zhang et al., 2012). Therefore, we wanted to investigate whether ZmMAP65-1a is involved in BR-induced antioxidant defence. To elucidate the relationship between ZmMAP65-1a and antioxidant defence, we used transient gene expression and transient RNA interference (RNAi) in maize mesophyll protoplasts. This approach has been proven to be efficient for functional analysis of plant genes (Sheen, 2001; Zhai et al., 2009; Kim and Somers, 2010).
The results showed that transient expression of ZmMAP65-1a in mesophyll protoplasts caused significant increases in the expression of ZmMAP65-1a and the antioxidant genes SOD4 and APX2 (Fig. 1C) when compared with that in protoplasts transfected with empty vector. Transient silencing of ZmMAP65-1a resulted in a marked reduction in the expression of ZmMAP65-1a and substantially decreased the gene expression of SOD4 and APX2 compared with the control (Fig. 1D). These results suggested that ZmMAP65-1a can induce the expression of antioxidant genes.
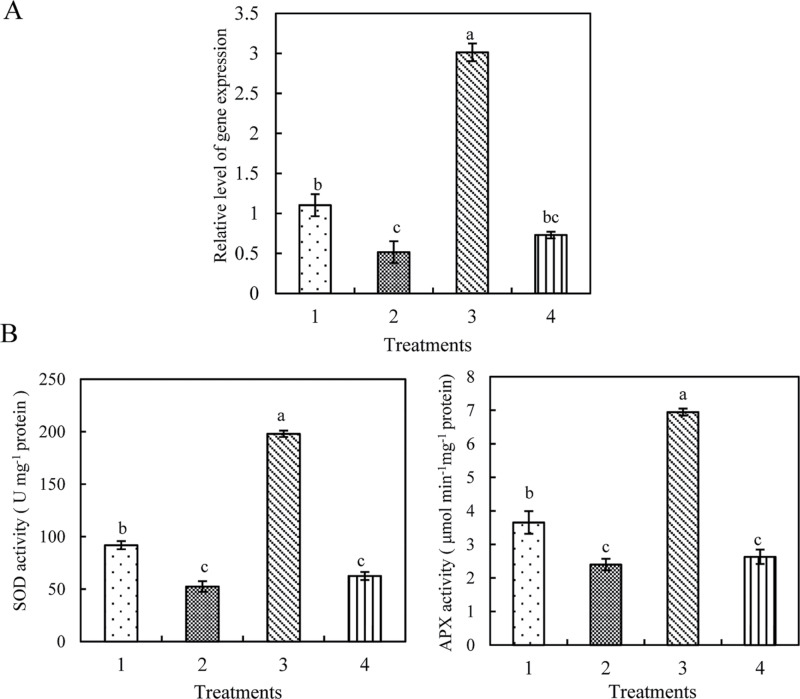
To investigate further the role of ZmMAP65-1a in BR-induced antioxidant defence, the activities of SOD and APX were determined. In agreement with the effects on gene expression, transient expression of ZmMAP65-1a in protoplasts also caused significant increases in the total activities of SOD and APX (Fig. 2C), and transient silencing of ZmMAP65-1a resulted in significant decreases in the activities of SOD and APX compared with the control (Fig. 3B). Furthermore, treatment with 10nM BR significantly induced the expression of ZmMAP65-1a (Figs 2B and 3A) and the activities of SOD and APX in control protoplasts (Figs 2A, C and 3B), which were further promoted in protoplasts transiently expressing ZmMAP65-1a (Fig. 2B, C). However, in protoplasts transiently silencing ZmMAP65-1a, BR treatment was no longer able to induce SOD and APX (Fig. 3). Taken together, these data demonstrated unequivocally that ZmMAP65-1a is required for BR-induced antioxidant defence in maize mesophyll protoplasts.
Fig. 2.
Transient expression of ZmMAP65-1a upregulates the activities of SOD and APX in protoplasts. (A) Time course of changes in the activities of the antioxidant enzymes SOD and APX in the protoplasts of maize leaves. Protoplasts were treated with 10nM BR for various times as indicated. Protoplasts treated with culture medium under the same conditions served as controls. (B) Expression analysis of ZmMAP65-1a in protoplasts. The protoplasts were treated as follows: 1, empty vector; 2, 35S–ZmMAP65-1a–YFP; 3, empty vector+BR; 4, 35S–ZmMAP65-1a–YFP+BR. The protoplasts were treated with 10nM BR for 10min, and the relative expression level of ZmMAP65-1a was analysed by real-time qRT-PCR. (C) Activities of SOD and APX in protoplasts transiently expressing ZmMAP65-1a. The protoplasts were treated as described in (B), and the activities of SOD and APX were measured. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P <0.05 according to Duncan’s multiple range test.
Fig. 3.
Transient silencing of ZmMAP65-1a downregulates the activities of SOD and APX in protoplasts. (A) Expression analysis of ZmMAP65-1a in protoplasts. The protoplasts were treated as follows: 1, distilled water; 2, RNAi; 3, distilled water+BR; 4, RNAi+BR. The protoplasts were treated with 10nM BR for 10min, and the relative expression level of ZmMAP65-1a was analysed by real-time qRT-PCR. (B) Activities of SOD and APX in protoplasts transiently silencing ZmMAP65-1a. The protoplasts were treated as described in (A), and the activities of SOD and APX were measured. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P <0.05 according to Duncan’s multiple range test.
Altered H2O2 levels affect the BR-induced expression of ZmMAP65-1a
As described above, BR induced the expression of ZmMAP65-1a. In addition, BR also induced H2O2 production, which subsequently enhanced antioxidant defence (Zhang et al., 2010). In order to reveal the relationship between ZmMAP65-1a and H2O2, the effect of treatment with 10mM H2O2 on the transcript level of ZmMAP65-1a was investigated. As shown in Fig. 4A, H2O2 treatment induced a significant increase in the expression of ZmMAP65-1a in maize leaves. Treatment with 10mM H2O2 induced a biphasic response, in which the first peak occurred after 20min of treatment, and the second peak appeared within 60min of treatment, in the expression of ZmMAP65-1a. Moreover, H2O2 treatment also rapidly induced the expression of ZmMAP65-1a in maize mesophyll protoplasts (Fig. 4B).
Fig. 4.
H2O2 is required for the BR-induced expression of ZmMAP65-1a in maize. (A, B) Expression analysis of ZmMAP65-1a in maize leaves (A) or mesophyll protoplasts (B) exposed to H2O2 treatment. The seedlings or protoplasts were treated with 10mM H2O2 (A) or 1mM H2O2 (B) for various times as indicated. Seedlings treated with distilled water and protoplasts treated with culture medium, under the same conditions during the whole period served as controls. The relative expression level of the ZmMAP65-1a gene was analysed by real-time qRT-PCR. (C) Effects of pre-treatments with ROS manipulators DMTU, DPI, and CAT on the expression of ZmMAP65-1a in response to BR treatment. The detached plants were pre-treated with 5mM DMTU, 100 μM DPI, or 200U CAT for 4h, and then exposed to 10nM BR treatment for 0.5h. Plants treated with distilled water under the same conditions served as a control. After treatment, the relative expression level of the ZmMAP65-1a gene was analysed by real-time qRT-PCR. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P <0.05 according to Duncan’s multiple range test.
In order to study the possible role of endogenous H2O2 induced by BR in the expression of ZmMAP65-1a, H2O2 scavengers, such as DMTU and CAT, were used. Furthermore, as NADPH oxidase is a key generator of H2O2 in plant cells (Xia et al. 2009), we also used DPI, an inhibitor of NADPH oxidase. Pre-treatments with DMTU, DPI, and CAT substantially reduced the BR-induced increase in the expression of ZmMAP65-1a in leaves, whereas the pre-treatments had little effect on the expression of ZmMAP65-1a in the absence of BR treatment (Fig. 4C). These data suggested that BR-induced H2O2 production is required for the BR-induced expression of ZmMAP65-1a in maize leaves.
ZmMAP65-1a affects BR-induced H2O2 production
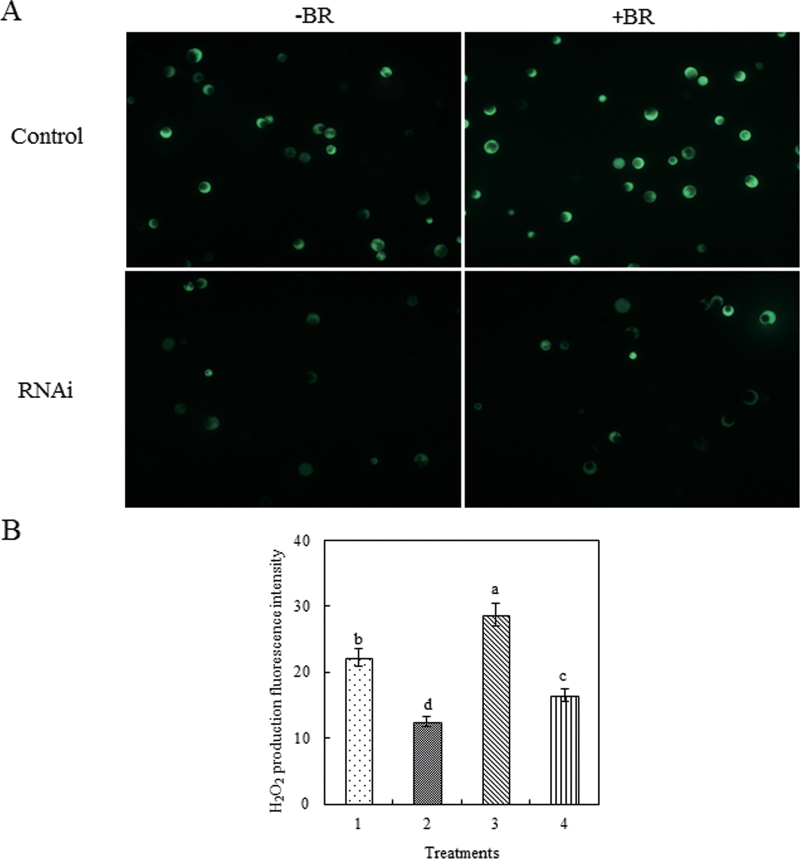
Crosstalk between H2O2 and other components, such as mitogen-activated protein kinase (MAPK), nitric oxide (NO) and calcium (Ca2+), has been demonstrated to operate in abscisic acid- or BR- induced antioxidant defence (Zhang et al., 2006, 2010; Sang et al., 2008). Therefore, the effect of BR-induced ZmMAP65-1a on BR-induced H2O2 production was investigated by determining the level of H2O2 production in response to BR in protoplasts transiently expressing or silencing ZmMAP65-1a. The results showed that transient expression of ZmMAP65-1a substantially increased H2O2 accumulation (Supplementary Fig. S1 at JXB online), while transient silencing of ZmMAP65-1a significantly reduced H2O2 accumulation (Fig. 5). BR treatment could further enhance the H2O2 level only in protoplasts transiently expressing ZmMAP65-1a (Supplementary Fig. S1) but not in protoplasts where ZmMAP65-1a was silenced (Fig. 5). These results suggested that ZmMAP65-1a is also required for BR-induced H2O2 production and that there is a crosstalk between H2O2 and ZmMAP65-1a in BR signalling.
Fig. 5.
Transient silencing of ZmMAP65-1a reduces BR-induced H2O2 production. (A) H2O2 fluorescence in protoplasts transiently silencing ZmMAP65-1a. Protoplasts transfected with dsRNA against ZmMAP65-1a (RNAi) or distilled water as a control were treated with 10nM BR (+BR) or incubation medium (–BR) for 10min and then loaded with H2DCF-DA for 10min. H2O2 was visualized by confocal microscopy. Experiments were repeated at least three times with similar results. (B) Quantitation of the fluorescence intensity in (A). The protoplasts were treated as follows: 1, distilled water; 2, RNAi; 3, distilled water+BR; 4, RNAi+BR. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P <0.05 according to Duncan’s multiple range test.
ZmMAP65-1a affects the expression of NADPH oxidase genes
NADPH oxidase is an important source of apoplastic H2O2 accumulation (Xia et al., 2009) and mediates H2O2 self-propagation in BR signalling (Zhang et al., 2010). To elucidate further the role of ZmMAP65-1a in the regulation of H2O2 accumulation in BR signalling, the expression of NADPH oxidase genes (rboh) was analysed. BR treatment induced significant increases in the expression of ZmrbohA, ZmrbohB, ZmrbohC, and ZmrbohD in protoplasts (Supplementary Fig. S2 at JXB online). RNAi silencing of ZmMAP65-1a in protoplasts reduced the expression of ZmrbohA, ZmrbohC, and ZmrbohD, and it could no longer be upregulated by BR treatment. In contrast, the BR-induced expression of ZmrbohB was only slightly decreased by ZmMAP65-1a silencing (Fig. 6A). These data suggested that ZmMAP65-1a is involved in the regulation of the gene expression of NADPH oxidase in BR signalling. However, transient expression of ZmMAP65-1a in protoplasts had little if any effect on the expression of ZmrbohA–D in either BR-treated or untreated protoplasts (Fig. 6B).
Fig. 6.
ZmMAP65-1a affects the expression of NADPH oxidase genes in protoplasts. (A) Expression analysis of ZmrbohA–D in protoplasts transiently silencing ZmMAP65-1a. Protoplasts were transfected with dsRNA against ZmMAP65-1a (RNAi) or with distilled water as a control. Protoplasts were treated with 10nM BR for 10min, and the relative expression levels of ZmrbohA–D were analysed by real-time qRT-PCR. (B) Expression analysis of ZmrbohA–D in protoplasts transiently expressing ZmMAP65-1a. Protoplasts were transfected with constructs carrying 35S–ZmMAP65-1a–YFP (ZmMAP65-1a), and control protoplasts were transfected with empty vector. Protoplasts were treated with 10nM BR for 10min and the relative expression levels of ZmrbohA–D were analysed by real-time qRT-PCR. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P <0.05 according to Duncan’s multiple range test.
ZmMPK5 interacting with ZmMAP65-1a promotes BR-induced antioxidant defence
ZmMPK5 is also required for the regulation of expression of NADPH oxidase gene in BR signalling in leaves of maize (Zhang et al., 2010). To determine whether there is a link between ZmMAP65-1a and ZmMPK5, the effect of ZmMPK5 on the expression of ZmMAP65-1a in BR signalling was tested. First, two inhibitors of MAPK kinase (MAPKK), PD98059 and U0126, which almost completely inhibit the activation of ZmMPK5 in response to BR (Zhang et al., 2010), were used. As shown in Fig. 7A, pre-treatment with PD98059 and U0126 strongly inhibited the BR-induced expression of ZmMAP65-1a in leaves but had no significant effect on the expression of ZmMAP65-1a in the absence of BR treatment. Next, the BR response was studied in protoplasts where ZmMPK5 was transiently silenced. Our results showed that BR treatment no longer caused increased expression of ZmMAP65-1a in protoplasts where ZmMPK5 was transiently silenced (Fig. 7B). These results suggested that BR-induced ZmMPK5 activation regulates ZmMAP65-1a at the transcriptional level.
Fig. 7.
ZmMPK5 regulates the expression of ZmMAP65-1a. (A) Effects of pre-treatment with the MAPKK inhibitors PD98059 and U0126 on the expression of ZmMAP65-1a in leaves of maize plants exposed to BR treatment. The detached plants were pre-treated with distilled water, 10 μM U0126 or 100 μM PD98059 for 4h, and then exposed to treatment with 10nM BR or distilled water for 30min. The relative expression level of ZmMAP65-1a was analysed by real-time qRT-PCR. (B) Expression analysis of ZmMAP65-1a in protoplasts transiently silencing ZmMPK5. Protoplasts were transfected with dsRNA against ZmMPK5 (RNAi) or distilled water as a control. Protoplasts were treated with 10nM BR for 10min, and the relative expression levels of ZmMPK5 and ZmMAP65-1a were analysed by real-time qRT-PCR. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P <0.05 according to Duncan’s multiple range test.
To investigate further the link between ZmMAP65-1a and ZmMPK5 in BR-induced antioxidant defence, both ZmMPK5 and dsRNA of ZmMAP65-1a were transfected into mesophyll protoplasts. The results showed that transient expression of ZmMPK5 alone significantly increased the activities of the antioxidant defence enzymes SOD and APX in control mesophyll protoplasts, which were further enhanced by BR treatment. However, the activities of SOD and APX were only partly upregulated in mesophyll protoplasts transfected with both ZmMPK5 and dsRNA of ZmMAP65-1a (Fig. 8). These results suggested that the interaction between ZmMAP65-1a and ZmMPK5 functions in BR-induced antioxidant defence.
Fig. 8.
The activities of SOD and APX in protoplasts transfected with 35S–ZmMPK5–YFP and dsRNA of ZmMAP65-1a. (A, B) The expression levels of ZmMAP65-1a (A) and ZmMAPK5 (B) in protoplasts. Protoplasts were treated as follows: 1, distilled water; 2, empty vector; 3, dsRNA of ZmMAP65-1a; 4, 35S–ZmMAPK5–YFP; 5, dsRNA of ZmMAP65-1a and 35S–ZmMAPK5–YFP. (C) The activities of the antioxidant enzymes SOD and APX in protoplasts treated as described in (A) and (B). Values are means ±SE of three different experiments. Means denoted by the same letter did not significantly differ at P <0.05 according to Duncan’s multiple range test.
ZmMAP65-1a interacts directly with ZmMPK5
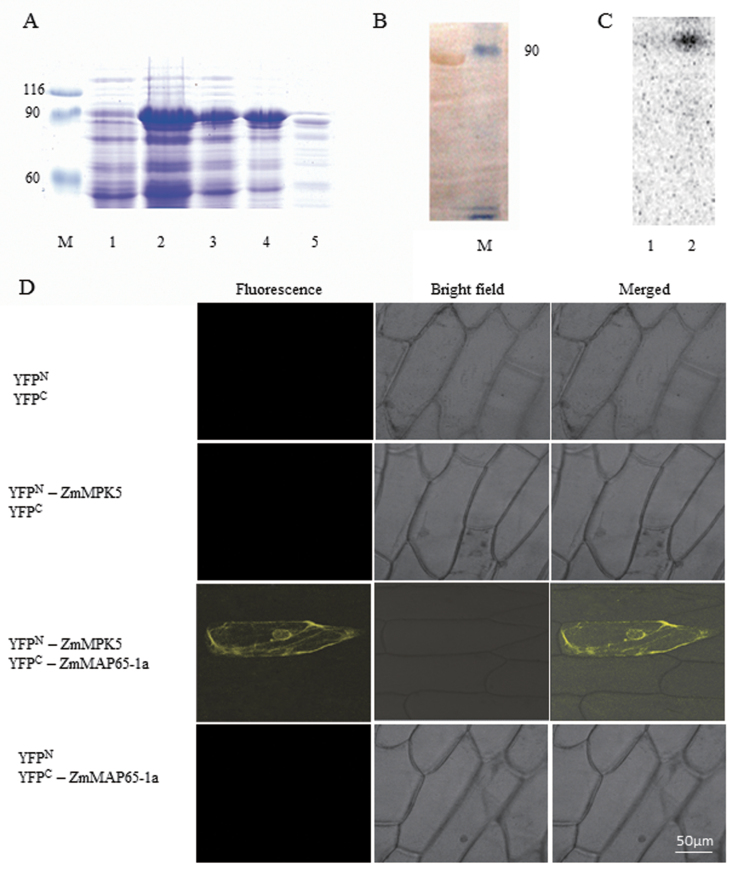
MAPKs have been shown to phosphorylate MAPs and the phosphorylated MAPs participated in many cell processes (Komis et al., 2011). To study whether there is a direct interaction between ZmMAP65-1a and ZmMPK5, the in vivo interaction between ZmMAP65-1a and ZmMPK5 was analysed by BiFC. In this system, YFP is split into N-terminal (YFPN) and C-terminal (YFPC) halves, and fluorescence is observed when two proteins fused to each YFP half interact with each other. Our experimental results showed that strong YFP fluorescence could be observed when YFPN–ZmMPK5 and YFPC–ZmMAP65-1a were co-expressed in onion epidermal cells (Fig. 9D). In contrast, no YFP signal was observed when no-fusion YFPC and no-fusion YFPN, YFPN–ZmMPK5 and no-fusion YFPC, and no-fusion YFPN and YFPC-ZmMAP65-1a, as the controls, were co-transformed. These results are consistent with an interaction between ZmMAP65-1a and ZmMPK5 in vivo.
Fig. 9.
ZmMPK5 interacts with ZmMAP65-1a. (A) Purification of GST-tagged ZmMAP65-1a. The fusion protein appeared as a major band of 90kDa by SDS–PAGE. M: Marker; 1, total extract from uninduced bacterial cells; 2, total extract from transformed bacterial cells after IPTG induction; 3, pellet from induced bacterial cells; 4, supernatant from induced bacterial cells; 5, Q-Sepharose elution of fusion protein. (B) Protein immunoblots probed with anti-GST antibody. Lane 1, GST–ZmMAP65-1a; Lane 2, Marker. (C) Phosphorylation of ZmMAP65-1a by ZmMPK5 in vitro. Protein extract from BR-treated leaves were immunoprecipitated with ZmMPK5 antibody. Protein prepared from uninduced (Lane 1) or IPTG induced (Lane 2) cultures was used as substrate and subjected to an in-gel kinase assay. (D) BiFC detection of ZmMAP65-1a and ZmMPK5 interaction in onion epidermal cells. Experiments were repeated at least three times with similar results.
To investigate whether ZmMPK5 can phosphorylate ZmMAP65-1a in vitro, immunocomplex kinase assays were performed using recombinant GST– ZmMAP65-1a as substrate. The GST–ZmMAP65-1a fusion protein with an apparent molecular mass of ~90kDa was expressed in E. coli and affinity purified (Fig. 9A). The same band was also detected with an anti-GST antibody (Fig. 9B). An antibody was raised against a peptide sequence in the C terminus of ZmMPK5 and used for the in vitro immunocomplex kinase assays. As shown in Fig. 9C, a strong phosphorylation band was detected, suggesting that ZmMPK5 directly phosphorylates ZmMAP65-1a in maize.
Discussion
MT organization and dynamics play a vital role in enhancing plant tolerance to abiotic stresses, such as drought, salt, and low temperature (Wang et al., 2011a ). MAP65 is one of the most abundant plant MT-associated proteins, and tightly regulates MT organization (Amos and Schlieper, 2005). In the presence of MAP65-1, microtubule bundles are more resistant to cold treatment in Arabidopsis (Mao et al., 2005). A recent study also revealed an important role of MAP65-1 in salt stress tolerance. Knockout of MAP65-1 results in microtubule depolymerization under salt stress and overexpression of MAP65-1 increass salt tolerance in Arabidopsis cells (Zhang et al., 2012). These results indicate an essential role of MAP65 in regulating MT organization in plant tolerance to abiotic stress.
Komorisono et al. (2005) reported a dwarf rice mutant with altered MT organization and upregulated gibberellin biosynthesis, suggesting a link between MTs and gibberellin signalling. More recent studies showed that the BR-induced antioxidant defence system enhanced plant tolerance to abiotic stress (Xia et al., 2009; Zhang et al., 2010). However, there is no report about a connection between MTs and BR signalling so far. Here, we discovered a novel function of MAP65 in BR-enhanced antioxidant defence. Exogenously applied BR upregulated the expression of ZmMAP65-1a in leaves (Fig. 1A) and mesophyll protoplasts of maize (Fig. 1B), suggesting that ZmMAP65-1a is very likely to participate in BR signalling. Furthermore, transient expression of ZmMAP65-1a in protoplasts significantly increased expression of the major antioxidant genes SOD4 and APX2 and the activities of the corresponding enzymes, which were further enhanced by BR treatment (Figs 1C and 2C). Conversely, compared with the control, RNAi silencing of ZmMAP65-1a in mesophyll protoplasts substantially decreased the expression of SOD4 and APX2 and the activities of SOD and APX, which could no longer be induced by BR treatment (Figs 1D and 3). These results indicate the crucial importance of ZmMAP65-1a in BR-induced antioxidant defence in leaves of maize plants.
H2O2 accumulation induced by various stimuli can induce antioxidant defence to scavenge abundant H2O2, protecting plants from damage (Miller et al., 2010). Here, we described the complex relationship between ZmMAP65-1a and H2O2 in BR signalling. Exogenous H2O2 treatment induced the expression of ZmMAP65-1a in both leaves and protoplasts (Fig. 4A, B). Scavenging or inhibiting the endogenous H2O2 level produced by BR inhibited the BR-induced increase in ZmMAP65-1a expression in maize leaves (Fig. 4C). These results suggest that H2O2 is required for the expression of ZmMAP65-1a in BR signalling. Previous work found that H2O2 treatment resulted in MT depolymerization in human cells (Sponne et al., 2003; Lee et al., 2005). Recently, Livanos et al. (2012) demonstrated that ROS signalling pathways are implicated in MT organization in plant cells, and they found that plant MTs are sensitive to both ROS overproduction and low ROS levels. Disturbance of ROS homeostasis induced atypical tubulin formation, which was a more stable structure than MTs, while MAP65-1, acting as a ‘tubulin-associated protein’, may underlie the bundling and/or the assembly of the atypical tubulin polymers. Taking these results together, it is possible that BR-produced H2O2 induces ZmMAP65-1a expression, subsequently regulating MT reorganization, leading to enhanced disturbance of ROS homeostasis tolerance. Our previous studies showed that there is crosstalk between H2O2 and NO, Ca2+/calmodulin and MAPK (Sang et al., 2008; Zhang et al., 2010). In the present study, crosstalk was detected between H2O2 and ZmMAP65-1a in BR signalling. BR-induced H2O2 accumulation also was regulated by transient expression and transient silencing of ZmMAP65-1a in maize mesophyll protoplasts (Fig. 5 and Supplementary Fig. S1). Thus, H2O2 appears to operate both upstream and downstream of ZmMAP65-1a, most likely indicating a positive feedback by ZmMAP65-1a on H2O2 production. A suggested model for the interaction between components described in this report is shown in Fig. 10.
Fig. 10.

Conceptual model of the role of ZmMAP65-1a in BR induce antioxidant defence. BR induces H2O2 production, the production of H2O2 increases ZmMAP65-1a accumulation, and ZmMPK5 phosphorylates ZmMAP65-1a, thus resulting in the upregulation of antioxidant defence enzymes. ZmMAP65-1a also enhances H2O2 production by NADPH oxidase, forming a positive amplification loop. The upregulation in the activities of antioxidant enzymes enhances the ability of cells to scavenge excess H2O2 under stress.
NADPH oxidase is a main source of BR-induced apoplastic H2O2 accumulation (Zhang et al., 2010). In the present study, BR treatment rapidly induced the expression of four NADPH oxidase genes in protoplasts (Supplementary Fig. S2). Transient RNAi silencing of ZmMAP65-1a in the protoplasts arrested the BR-induced upregulation in the expression of NADPH oxidase genes (Fig. 6A). Considering the crosstalk between BR-induced H2O2 accumulation and BR-induced ZmMAPK65-1a expression described above, we propose that ZmMAP65-1a induced by BR-produced H2O2 increases NADPH oxidase gene expression, which in turn enhances H2O2 accumulation, forming a H2O2 self-propagation loop, in BR signalling in leaves of maize plants.
Surprisingly, transient expression of ZmMAP65-1a did not enhance the expression of the NADPH oxidase genes, except for a slight increase for ZmrbohC (Fig. 6B). One possible explanation for this disparity is that BR treatment induces not only the expression of the ZmMAP65-1a gene but also modification of the ZmMAP65-1a protein, and modification of ZmMAP65-1a is essential for the regulation of NADPH oxidase gene expression in BR signalling. A similar pattern of post-translational regulation has been reported for the Arabidopsis and tobacco homologues AtMAP65-1, AtMAP65-3, and NtMAP65-1a (Smertenko et al., 2004; Sasabe et al., 2006; Caillaud et al., 2008). Besides that, in addition to NADPH oxidases, cell-wall peroxidase and polyamine oxidase are also sources of apoplastic H2O2 production (Mittler, 2002). Moreover, the chloroplast is another location of BR-induced H2O2 production (Zhang et al., 2010). BR-induced H2O2 production from these different sources is not synchronous with that from NADPH oxidase, as described by Lin et al. (2009). Therefore, transient expression of ZmMAP65-1a may induce H2O2 production from other sources and thereby cause the upregulation of antioxidant defence enzymes in BR signalling.
Recent studies demonstrated a link between BR and MAPK. Both BR signalling and MAPK regulated the transcription factor SPEECHLESS in stomatal development in Arabidopsis (Gudesblat et al., 2012). AtMKK4 and AtMKK5 acted downstream of BR signalling as targets of the BIN2 kinase (Khan et al., 2013). BR regulated stomatal development by activating the MAPK cascade (Kim et al., 2012). Inhibiting the expression (Nie et al., 2013) and activity (Zhang et al., 2010) of MAPK reduced H2O2 accumulation and the activities of antioxidant defence enzymes in BR signalling. Moreover, a recent study revealed that MAPK is involved in MT rearrangements, probably via MAP65-1 (Beck et al., 2011). Here, we connected the MAPK, MAP65, and BR signalling. Our experimental results showed that inhibiting the activity and expression of ZmMPK5 by pre-treatments with inhibitors or RNAi silencing blocked the BR-induced expression of ZmMAP65-1a (Fig. 7). Furthermore, transient expression of ZmMPK5 could increase the activities of SOD and APX, but this effect was partly blocked by transient silencing of ZmMAP65-1a (Fig. 8). These data indicate that ZmMAP65-1a and ZmMPK5 need to interact to assert their role in BR-induced antioxidant defence. Obviously, the interaction between ZmMAP65-1a and ZmMPK5 might modulate other process as well.
Previous studies have shown that MAPKs phosphorylate a variety of substrates including transcription factors, other protein kinases, and cytoskeleton-associated proteins in response to various stimuli (Nakagami et al., 2005; Pitzschke and Hirt, 2006). MAP65 phosphorylation by MAPK was found to affect its MT bundling activity during cytokinesis and interphase (Sasabe and Machida, 2006, 2012). An MAPK mutant showed prominent cytokinetic defects (Beck et al., 2011), suggesting an important role of MAPK phosphorylation in cytokinesis. Some studies have showed that MAP65-1 from Arabidopsis is phosphorylated by MPK4 and MPK6 (Smertenko et al., 2006; Beck et al., 2010), while MAP65-2 and MAP65-3 are phosphorylated by the heterologous protein in tobacco, NRK1 (Komis et al., 2011). However, there is no evidence as to whether MAP65 is also phosphorylated by MAPK in plant response to BR or stress. The present study not only revealed the in vivo protein interaction between ZmMPK5 and ZmMAP65-1a but also provided evidence of phosphorylation of ZmMAP65-1a by ZmMPK5 (Fig. 9). The phosphorylation of MAP65 by MAPK renders it incapable of MT bundling and enhances destabilization and turnover of MTs at the phragmoplast equator in mitosis (Sasabe et al., 2006; Smertenko et al., 2006). During salt stress, both depolymerization and reorganization of MT are believed to play a vital role in the stress response (Wang et al., 2011b). Under oxidative stress, ROS overproduction induces disruption of MTs and the formation of atypical tubulin polymers, which is a common adaptation and protection against stress (Livanos et al., 2012). Thus, these studies and our results suggest that BR induces H2O2 accumulation, which then results in depolymerization of MTs and atypical tubulin polymer formation, which is promoted by ZmMPK5 phosphorylation of ZmMAP65-1a (Fig. 10). Besides phosphorylation of MAP65-1, Zhang et al. (2012) found that phosphatidic acid binds to MAP65-1, increasing its activity in enhancing MT polymerization and bundling, thereby enhancing salt stress tolerance. Therefore, there should be multiple ways to regulation MAP65 activity in modulating the depolymerization and reorganization of MTs during stresses.
In summary, our experimental results indicate that ZmMAP65-1a is required for BR-induced antioxidant defence. In this process, BR-induced expression of ZmMAP65-1a is mediated by BR-induced H2O2 production. Conversely, the increase in the expression of ZmMAP65-1a amplifies H2O2 production via induction of NADPH oxidase genes, forming a positive H2O2 amplification loop. ZmMPK5 regulates ZmMAP65-1a gene expression and phosphorylation in BR signalling. Our results clearly suggest that ZmMAP65-1a is an important component in BR-induced antioxidant defence in maize. Whether there are other kinases or signal transduction pathways that regulate MAP65 should be addressed in future studies.
Supplementary data
Supplementary data are available at JXB online
Fig. S1. Transient expression of ZmMAP65-1a enhances BR-induced H2O2 production.
Fig. S2. Time course of changes in the expression of NADPH oxidase genes in response to BR treatment.
Acknowledgments
This work was supported by the National Basic Research Program of China (grant no. 2012CB114300 to M.J.), the National Natural Science Foundation of China (30971712 and 31071344 to A.Z. and 31271631, 30970238, and 31070254 to M.J.), the Natural Science Foundation of Jiangsu Province (BK2010455 to A.Z.), the Fundamental Research Funds for the Central Universities (KYZ201157 to A.Z. and KYZ200905, and KYT201001 to M.J.), the Program for New Century Excellent Talents in University (NCET-10–0498 to A.Z.), and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Glossary
Abbreviations:
- APX
ascorbate peroxidase
- BiFC
bimolecular fluorescence complementation
- BR
brassinosteroid
- CAT
catalse
- DMTU
dimethylthioure
- DPI
diphenylene iodonium
- GST
glutathione S-transferase
- MAP
microtubule-associated protein
- MAPK
mitogen-activated protein kinase
- MT
microtubule
- NO
nitric oxide
- qRT-PCR
quantitative reverse transcriptase-PCR
- RNAi
RNA interference
- ROS
reactive oxygen species
- SE
standard error
- SOD
superoxide dismutase
- YFP
yellow fluorescent protein.
References
- Amos LA, Schlieper D. 2005. Microtubules and maps. Advances in Protein Chemistry 71, 257–298. [DOI] [PubMed] [Google Scholar]
- Bajguz A. 2007. Metabolism of brassinosteroids in plants. Plant Physiology and Biochemistry 45, 95–107. [DOI] [PubMed] [Google Scholar]
- Beck M, Komis G, Müller J, Menzel D, Šamaj J. 2010. Arabidopsis homologs of nucleus- and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization. Plant Cell 22, 755–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Komis G, Ziemann A, Menzel D, Šamaj J. 2011. Mitogen-activated protein kinase 4 is involved in the regulation of mitotic and cytokinetic microtubule transitions in Arabidopsis thaliana . New Phytologist 189, 1069–1083. [DOI] [PubMed] [Google Scholar]
- Berberich T, Sano H, Kusano T. 1999. Involvement of a MAP kinase, ZmMPK5, in senescence and recovery from low-temperature stress in maize. Molecular and General Genetics 262, 534–542. [DOI] [PubMed] [Google Scholar]
- Bishop GJ, Koncz C. 2002. Brassinosteroids and plant steroid hormone signaling. Plant Cell 14, S97–S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. 2006. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. The Plant Journal 45, 113–122. [DOI] [PubMed] [Google Scholar]
- Caillaud MC, Lecomte P, Jammes F, et al. 2008. MAP65-3 microtubule-associated protein is essential for nematode-induced giant cell ontogenesis in Arabidopsis . Plant Cell 20, 423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary SP, Bhardwaj R, Gupta BD, Dutt P, Gupta RK, Biondi S, Kanwar M. 2010. Epibrassinolide induces changes in indole-3-acetic acid, abscisic acid and polyamine concentrations and enhances antioxidant potential of radish seedlings under copper stress. Physiologia Plantarum 140, 280–296. [DOI] [PubMed] [Google Scholar]
- Choudhary SP, Kanwar M, Bhardwaj R, Gupta BD, Gupta RK. 2011. Epibrassinolide ameliorates Cr (VI) stress via influencing the levels of indole-3-acetic acid, abscisic acid, polyamines and antioxidant system of radish seedlings. Chemosphere 84, 592–600. [DOI] [PubMed] [Google Scholar]
- Choudhary SP, Yu JQ, Yamaguchi-Shinozaki K, Shinozaki K, Tran, Lam-Son P. 2012. Benefits of brassinosteroid crosstalk. Trends in Plant Science 10, 594–605. [DOI] [PubMed] [Google Scholar]
- Clouse SD, Sasse JM. 1998. Brassinosteroids: essential regulators of plant growth and development. Annual Review of Plant Physiology and Plant Molecular Biology 49, 427–451. [DOI] [PubMed] [Google Scholar]
- Cui JX, Zhou YH, Ding JG, Xia XJ, Shi K, Chen SC, Asami T, Chen Z, Yu JQ. 2011. Role of nitric oxide in hydrogen peroxide-dependent induction of abiotic stress tolerance by brassinosteroids in cucumber. Plant, Cell & Environment 34, 347–358. [DOI] [PubMed] [Google Scholar]
- Desai A, Mitchison TJ. 1997. Microtubule polymerization dynamics. Annual Review of Cell and Developmental Biology 13, 83–117. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Weits DA, Cruz-Ramirez A, et al. 2012. A PLETHORA-auxin transcription module controls cell division plane rotation through MAP65 and CLASP. Cell 149, 383–396. [DOI] [PubMed] [Google Scholar]
- Divi UK, Krishna P. 2009. Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. New Biotechnology 26, 131–136. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17, 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat G, Schneider-Pizon J, Betti C, et al. 2012. SPEECHLESS integrates brassinosteroid and stomata signaling pathways. Nature Cell Biology 14, 548–554. [DOI] [PubMed] [Google Scholar]
- Guo L, Ho CMK, Kong Z, Lee YRJ, Qian Q, Liu B. 2009. Evaluating the microtubule cytoskeleton and its interacting proteins in monocots by mining the rice genome. Annals of Botany 103, 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey PJ, Hawkins TJ, Igarashi H, Kaloriti D, Smertenko A. 2002. The plant cytoskeleton: recent advances in the study of the plant microtubule-associated proteins MAP-65, MAP-190 and the Xenopus MAP215-like protein, MOR1. Plant Molecular Biology 50, 915–924. [DOI] [PubMed] [Google Scholar]
- Jiang CJ, Sonobe S. 1993. Identification and preliminary characterization of a 65kDa higher-plant microtubule-associated protein. Journal of Cell Science 105, 891–901. [DOI] [PubMed] [Google Scholar]
- Kagale S, Divi UK, Krochko JE, Keller WA, Krishna P. 2007. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225, 353–364. [DOI] [PubMed] [Google Scholar]
- Keech O, Pesquet E, Gutierrez L, Ahad A, Bellini C, Smith SM, Gardeström P. 2010. Leaf senescence is accompanied by an early disruption of the microtubule network in Arabidopsis . Plant Physiology 154, 1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Rozhon W, Biqeard J, et al. 2013. Brassinosteroid-regulated GSK3/Shaggy-like kinases phosphorylate mitogen-activated protein (MAP) kinase kinases, which control stomata development in Arabidopsis thaliana . Journal of Biological Chemistry 288, 7519–7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Somers DE. 2010. Rapid assessment of gene function in the circadian clock using artificial microRNA in Arabidopsis thaliana mesophyll protoplasts. Plant Physiology 154, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Michniewicz M, Bergmann DC, Wang ZY. 2012. Brassinosteroid regulates stomatal development by GSKS-mediated inhibition of a MAPK pathway. Nature 482, 419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komis G, Illés P, Beck M, Šamaj J. 2011. Microtubules and mitogen-activated protein kinase signalling. Current Opinion in Plant Biology 14, 650–657. [DOI] [PubMed] [Google Scholar]
- Komorisono M, Ueguchi-Tanaka M, Aichi I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M, Sazuka T. 2005. Analysis of the rice mutant dwalf and gladius leaf 1. Aberrant katanin-mediated microtubule organization cause up-regulation of gibberellin biosynthetic genes independently of gibberellin signaling. Plant Physiology 138, 1982–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza M, Garcia-Ponce B, Castrillo G, et al. 2012. Role of actin cytoskeleton in brassinosteroid signaling and in its integration with the auxin response in plants. Developmental Cell 22, 1275–1285. [DOI] [PubMed] [Google Scholar]
- Lee CF, Liu CY, Hsieh RH, Wei YH. 2005. Oxidative stress-induced depolymerization of microtubules and alteration of mitochondrial mass in human cells. Annals of the New York Academy of Sciences 1042, 246–254. [DOI] [PubMed] [Google Scholar]
- Lin F, Ding H, Wang J, Zhang H, Zhang A, Zhang Y, Tan M, Dong W, Jiang M. 2009. Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signalling. Journal of Experimental Botany 60, 3221–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livanos P, Galatis B, Quader H, Apostolakos P. 2012. Disturbance of reactive oxygen species homeostasis induces atypical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana . Cytoskeleton 69, 1–21. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Courtney S, Hassfurder M, Dhingra S, Bryant A, Shaw SL. 2011. Microtubule-associated proteins MAP65-1 and MAP65-2 positively regulate axial cell growth in etiolated Arabidopsis hypocotyls. Plant Cell 23, 1889–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü B, Gong Z, Wang J, Zhang J, Liang J. 2007. Microtubule dynamics in relation to osmotic stress-induced ABA accumulation in Zea mays roots. Journal of Experimental Botany 58, 2565–2572. [DOI] [PubMed] [Google Scholar]
- Ma F, Lu R, Liu H, Shi B, Zhang J, Tan M, Zhang A, Jiang M. 2012. Nitric oxide-activated calcium/calmodulin-dependent protein kinase regulates the abscisic acid-induced antioxidant defence in maize. Journal of Experimental Botany 63, 4835–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, Jin L, Li H, Liu B, Yuan M. 2005. Two microtubule-associated proteins of the Arabidopsis MAP65 family function differently on microtubules. Plant Physiology 138, 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G, Buschmann H, Doonan JH, Lloyd CW. 2006. The role of MAP65-1 in microtubule bundling during Zinnia tracheary element formation. Journal of Cell Science 119, 753–758. [DOI] [PubMed] [Google Scholar]
- Mathur J, Chua NH. 2000. Microtubule stabilization leads to growth reorientation in Arabidopsis trichomes. Plant Cell 12, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. 2010. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell & Environment 33, 453–467. [DOI] [PubMed] [Google Scholar]
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7, 405–410. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Pitzschke A, Hirt H. 2005. Emerging MAP kinase pathways in plant stress signaling. Trends in Plant Science 10, 339–346. [DOI] [PubMed] [Google Scholar]
- Nie WF, Wang MM, Xia XJ, Zhou YH, Shi K, Chen ZX, Yu JQ. 2013. Silencing of tomato RBOH1 and MPK2 abolishes brassinosteroid-induced H2O2 generation and stress tolerance. Plant, Cell & Environment 36, 789–803. [DOI] [PubMed] [Google Scholar]
- Nogales E. 2000. Structural insight into microtubule function. Annual Review of Biochemisty 69, 277–302. [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Hirt H. 2006. Mitogen-activated protein kinase and reactive oxygen species signaling in plants. Plant Physiology 141, 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang J, Zhang A, Lin F, Tan M, Jiang M. 2008. Cross-talk between calcium-calmodulin and nitric oxide in abscisic acid signaling in leaves of maize plants. Cell Research 18, 577–588. [DOI] [PubMed] [Google Scholar]
- Sasabe M, Machida Y. 2006. MAP65: a bridge linking a MAP kinase to microtubule turnover. Current Opinion in Plant Biology 9, 563–570. [DOI] [PubMed] [Google Scholar]
- Sasabe M, Machida Y. 2012. Regulation of organization and function of microtubules by the mitogen-activated protein kinase cascade during plant cytokinesis. Cytoskeleton 69, 913–918. [DOI] [PubMed] [Google Scholar]
- Sasabe M, Soyano T, Takahashi Y, Sonobe S, Igarashi H, Itoh TJ, Hidaka M, Machida Y. 2006. Phosphorylation of NtMAP65-1 by a MAP kinase downregulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes and Development 20, 1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook JC, Kaloriti D. 2008. Microtubules, MAPs and plant directional cell expansion. Trends in Plant Science 13, 303–310. [DOI] [PubMed] [Google Scholar]
- Sheen J. 2001. Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiology 127, 1466–1475. [PMC free article] [PubMed] [Google Scholar]
- Smertenko AP, Chang HY, Wagner V, Kaloriti D, Fenyk S, Sonobe S, Lloyd C, Hauser MT, Hussey PJ. 2004. The Arabidopsis microtubule-associated protein AtMAP65-1: molecular analysis of its microtubule bundling activity. Plant Cell 16, 2035–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko AP, Chang HY, Sonobe S, Fenyk SI, Weingartner M, Bögre L, Hussey PJ. 2006. Control of the AtMAP65-1 interaction with microtubules through the cell cycle. Journal of Cell Science 119, 3227–3237. [DOI] [PubMed] [Google Scholar]
- Soares VLF, Rodrigues SM, de Oliveira TM, et al. 2011. Unraveling new genes associated with seed development and metabolism in Bixa orellana L. by expressed sequence tag (EST) analysis. Molecular Biology Reports 38, 1329–1340. [DOI] [PubMed] [Google Scholar]
- Sponne I, Fifre A, Drouet B, Klein C, Koziel V, Pincon-Raymond M, Olivier JL, Chambaz J, Pillot T. 2003. Apoptotic neuronal cell death induced by the non-fibrillar amyloid-β peptide proceeds through an early reactive oxygen species-dependent cytoskeleton perturbation. Journal of Biological Chemisty 278, 3437–3445. [DOI] [PubMed] [Google Scholar]
- Tan M, Lu J, Zhang A, Hu B, Zhu X, Li W. 2011. The distribution and cooperation of antioxidant (iso)enzymes and antioxidants in different subcellular compartments in maize leaves during water stress. Journal of Plant Growth Regulation 30, 255–271. [Google Scholar]
- Wang C, Zhang LJ, Chen WF. 2011a. Plant cortical microtubules are putative sensors under abiotic stresses. Biochemistry 76, 320–326. [DOI] [PubMed] [Google Scholar]
- Wang S, Kurepa J, Hashimoto T, Smalle JA. 2011b. Salt stress-induced disassembly of Arabidopsis cortical microtubule arrays involves 26S proteasome-dependent degradation of SPIRAL1. Plant Cell 23, 3412–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY. 2012. Brassinosteroids modulate plant immunity at multiple levels. Proceedings of the National Academy of the Sciences, USA 109, 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ. 2009. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiology 150, 801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Sooksa-nguan T, Vatamaniuk OK. 2009. Establishing RNA interference as a reverse-genetic approach for functional analysis in protoplasts. Plant Physiology 149, 642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Jiang M, Zhang J, Tan M, Hu X. 2006. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiology 141, 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Zhang J, Ye N, Cao J, Tan M, Zhang J, Jiang M. 2010. ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize. Journal of Experimental Botany 61, 4399–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Zhang J, Zhang J, Ye N, Zhang H, Tan M, Jiang M. 2011. Nitric oxide mediates brassinosteroid-induced ABA biosynthesis involved in oxidative stress tolerance in maize leaves. Plant and Cell Physiology 52, 181–192. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lin F, Mao T, Nie J, Yan M, Yuan M, Zhang W. 2012. Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis . Plant Cell 24, 4555–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.