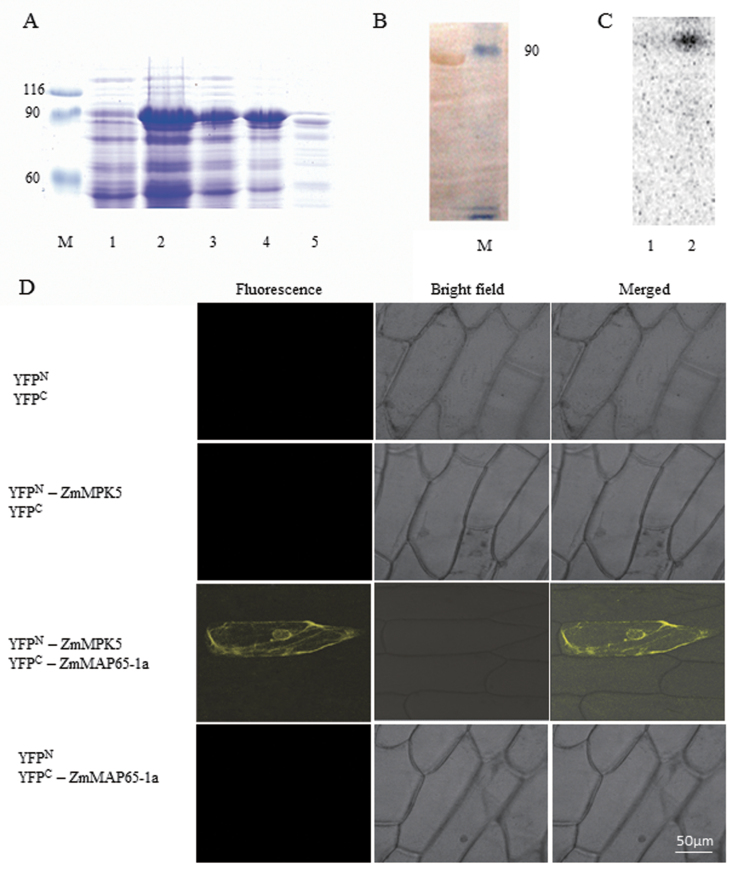
Fig. 9.
ZmMPK5 interacts with ZmMAP65-1a. (A) Purification of GST-tagged ZmMAP65-1a. The fusion protein appeared as a major band of 90kDa by SDS–PAGE. M: Marker; 1, total extract from uninduced bacterial cells; 2, total extract from transformed bacterial cells after IPTG induction; 3, pellet from induced bacterial cells; 4, supernatant from induced bacterial cells; 5, Q-Sepharose elution of fusion protein. (B) Protein immunoblots probed with anti-GST antibody. Lane 1, GST–ZmMAP65-1a; Lane 2, Marker. (C) Phosphorylation of ZmMAP65-1a by ZmMPK5 in vitro. Protein extract from BR-treated leaves were immunoprecipitated with ZmMPK5 antibody. Protein prepared from uninduced (Lane 1) or IPTG induced (Lane 2) cultures was used as substrate and subjected to an in-gel kinase assay. (D) BiFC detection of ZmMAP65-1a and ZmMPK5 interaction in onion epidermal cells. Experiments were repeated at least three times with similar results.