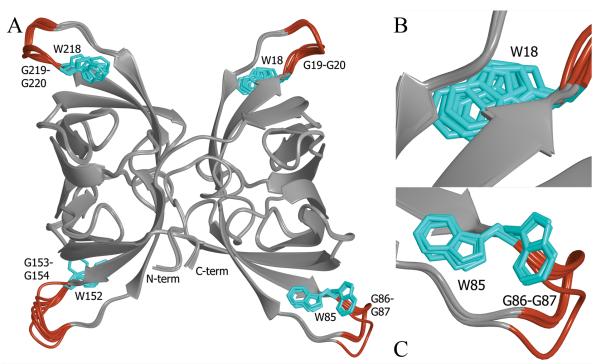
FIGURE 6.
Superposition of the eight noncrystallographically related BOA molecules found in the asymmetric unit of the ligand-free crystal. The only noticeable areas of conformational heterogeneity are shaded in red, and these loop regions contain the conserved WGG motif involved in binding the ligand in each binding site (panel A). Panels B and C provide enlarged views of the key tryptophan side chain (colored cyan) in two different binding sites. Trp18 (panel B) exhibits some conformational variability within a single rotameric state in the absence of ligand. This also holds for Trp218. Both Trp85 (panel C) and Trp152 adopt two distinct rotamers.