FIGURE 2.
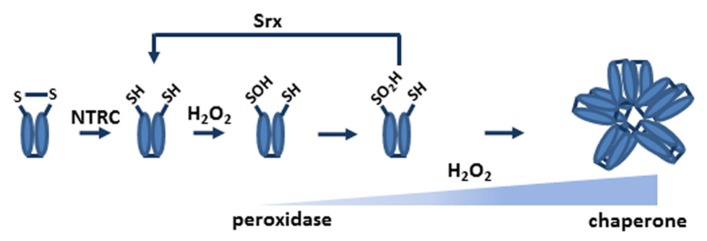
NTRC and Srx determine the redox status of chloroplast 2-Cys Prxs. Under oxidant conditions, the sulfenic acid intermediate of the peroxidatic cysteine residue may be further oxidized to sulfinic acid. The reduction of the enzyme, which is most efficiently performed by NTRC, is a pre-requisite for sulfenic acid formation and, thus, for overoxidation. Srx is able to catalyze the reversion of the overoxidized to the reduced form of the enzyme. Therefore, the redox status of chloroplast 2-Cys Prxs is highly dependent of NTRC and Srx. The quaternary structure of 2-Cys Prxs determines the activity of these enzymes. In the reduced form the enzyme is a dimer and shows peroxidase activity; overoxidation favors the formation of the decameric form, which lacks peroxidase activity and shows chaperone activity.
