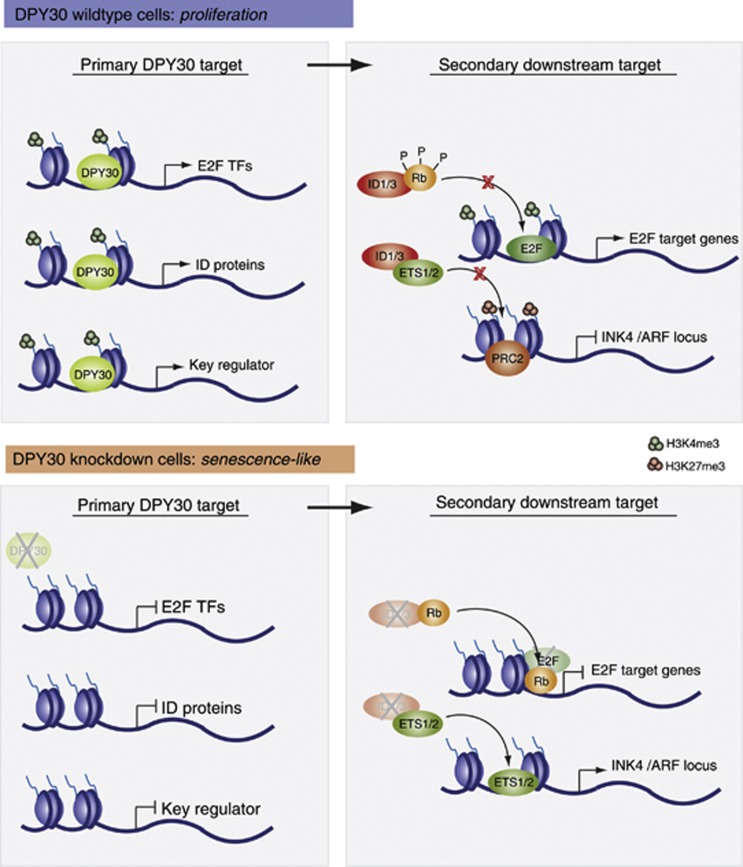
Figure 6.
Potential molecular mechanisms involved in the senescence-like phenotype induced by the loss of DPY30. In DPY30 wild-type cells, expression of various target genes is under control of a functionally active DPY30-Set1/MLL complex that ensures proper H3K4me3 on these genes. This leads to the expression of the E2F transcription factors and ID proteins (among others), which are involved in regulating secondary downstream target genes: E2F binds and activates transcription of genes involved in cell-cycle progression, while the ID proteins negatively influence the action of other transcription factors, such as Rb and ETS1/2. As a consequence, Rb cannot act as a repressor at E2F target genes, and ETS1/2 cannot activate the expression of the INK4/ARF locus, which is required for the cell to proliferate. In DPY30 knockdown cells, expression of various target genes is repressed (including E2F transcription factors and ID proteins), which correlates with impaired H3K4me3 levels. Expression of E2F target genes is thus shut down since, first, E2F transcription factors are absent, and second, hypophosphorylated Rb is no longer blocked by ID proteins and can repress E2F target genes. Additionally, ETS1/2 transcription factors are free to activate the INK4/ARF locus, which contributes to a senescence-like phenotype. In addition to the E2F transcription factors and ID proteins, other key regulators could be under the control of DPY30. Upon depletion of DPY30, these key regulators would be deregulated, leading to secondary downstream effects that could also play a role in senescence pathways.