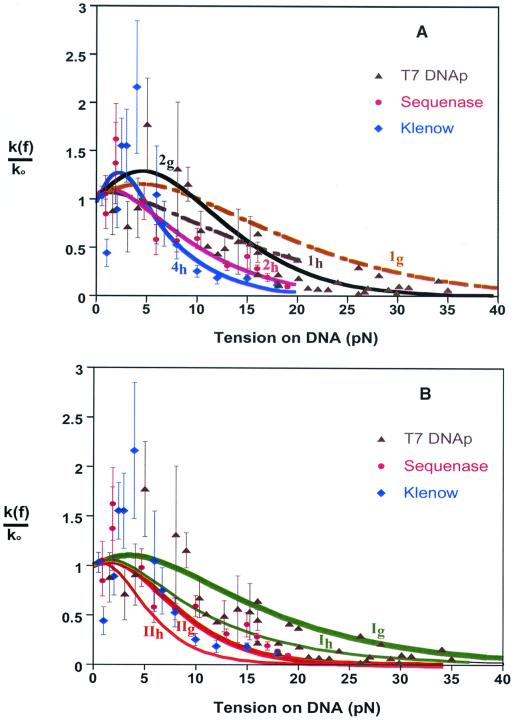
Figure 3.
Model calculations and experimental data for force-dependent replication rates catalyzed by three enzymes. Ordinate is normalized to rate at zero force, k0. Data for T7 DNA polymerase (k0 = 130 bases/sec) (▴) from ref. 10, for Sequenase (k0 = 200 bases/sec) (●) and Klenow fragment (k0 = 13.5 bases/sec) (⧫) from ref. 11. (A) Dashed curves from Eq. 1 with n = 1 and Δq either the Gibbs free energy (1g, ref. 10) or the enthalpy (1h, ref. 11), as derived from experimental force-extension curves for ss- and dsDNA.** Full curves show fits to data obtained with n = 2 for T7 DNAp (2g, ref. 10) and Sequenase (2h, ref. 11), and with n = 4 for Klenow fragment (4h, ref. 11). (B) Model curves from Eq. 3 for free-energy and enthalpy variants (subscripts g and h) of limiting Cases I and II; all pertain to n = 1 and the same local contour length and stiffness parameters¶¶ for the three enzymes.