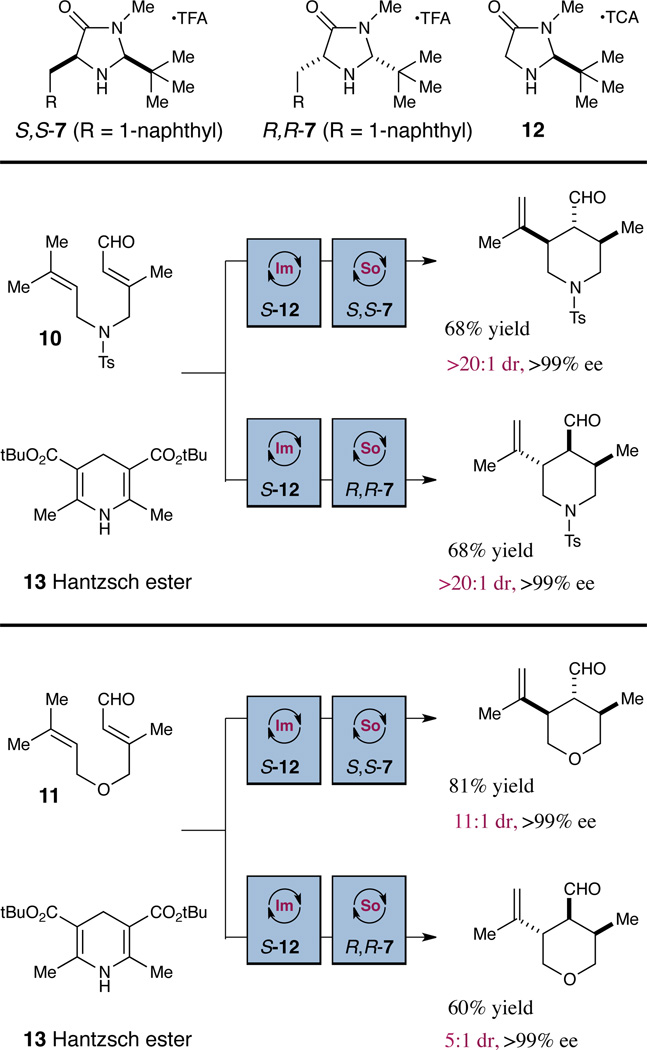
Scheme 1.
Sequential organocatalytic hydrogenation-cyclizationa
Conditions: Step 1) 2.5 equiv. 13, 20 mol% 12, CHCl3, −50 °C, 24 hours. Step 2) 2.5 equiv. Fe(phen)3(Tf2N)3, 2.5 equiv. Na2HPO4, 20 mol % 7, −30 °C, DME, 12 hours. aDiastereoselectivities determined by 1H-NMR analysis. Enantiomeric excess determined by chiral HPLC analysis of the corresponding alcohol or terephthalic acid monomethyl diester.