Table 2.
Scope of dual activation formal [4+3] annulationa
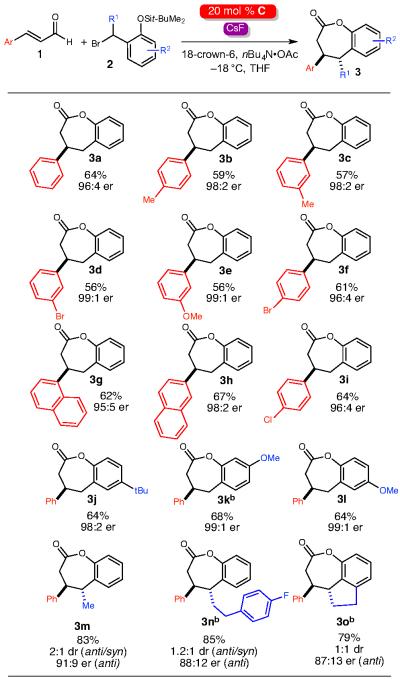
|
Reactions were performed at 0.4 mmol with 1 equiv of 1, 2 equiv of 2, 0.3 equiv base, 2 equiv CsF and 2 equiv crown ether in THF (0.15 M in 1). Isolated yields of 3 are reported. Er was determined by chiral stationary-phase high performance liquid chromatography (HPLC).
Benzylic chloride was used for 2 instead of benzylic bromide.
