Abstract
Objectives
The aim of this pilot study was to explore possible ultrasound parameters for the early detection of alcohol-mediated fetal somatic and central nervous system (CNS) maldevelopment. Maternal alcohol ingestion during pregnancy may lead to fetal alcohol spectrum disorders (FASD), which encompass a broad range of structural abnormalities including growth impairment, specific craniofacial features and CNS abnormalities. Early detection of fetuses at risk of FASD would support earlier interventions.
Methods
We performed a longitudinal prospective pilot study from 2004 to 2006 at two sites in Ukraine. A sample of pregnant women who reported consuming moderate-to-heavy amounts of alcohol participated in a comprehensive maternal interview, and received ultrasound evaluation of fetal growth and specific fetal brain measurements during the second and third trimesters. These measurements were compared with those collected from a group of pregnant women who consumed little-to-no alcohol during pregnancy, and who were recruited and followed in the same manner.
Results
From 6745 screened women, 84 moderate-to-heavy alcohol users and 82 comparison women were identified and ultrasound examinations performed. After controlling for maternal smoking, alcohol-exposed fetuses had shorter mean femur length, caval–calvarial distance and frontothalamic measurements in the second trimester (P < 0.05), and alcohol-exposed fetuses also had shorter frontothalamic distance measurements in the third trimester relative to comparison fetuses (P < 0.05). In addition, after controlling for maternal smoking, both mean orbital diameter and biparietal diameter measurements were significantly smaller on average in the alcohol-exposed group in the third trimester relative to comparison fetuses (P < 0.05).
Conclusions
Significant differences in selected somatic and brain measurements were noted between alcohol-exposed and comparison fetuses, suggesting these markers may be further explored for clinical utility in prenatal identification of affected children. Further study correlating these findings with alcohol-related physical features of the newborn and subsequent comparisons of neuro-developmental outcomes will help define potential uses of prenatal ultrasound for intervention and prevention of FASD.
Keywords: brain measurement, frontothalamic distance, prenatal alcohol exposure, ultrasound
INTRODUCTION
One of the greatest challenges facing clinicians working in the area of fetal alcohol spectrum disorders (FASD) is the early and accurate recognition of affected individuals in order to facilitate earlier intervention and prevention. The criteria for diagnosis of fetal alcohol syndrome (FAS) are well established when based on dysmorphic features identified postnatally, and include characteristic abnormalities of craniofacial development as well as growth deficiency and neurobehavioral impairment1,2. However, there are many individuals who fall into the spectrum of FASD who do not exhibit the classical features of FAS but whose prenatal alcohol exposure has adversely affected brain development3, with an estimated prevalence in the general population of approximately 1%4. These alcohol-associated brain abnormalities include aplasia or hypoplasia of the corpus callosum, cerebellar abnormalities with either global hypoplasia or vermian dysgenesis5,6 and reduced cortical thickness7. Of particular note, although the entire cortex may be adversely affected by alcohol, the most profound changes are seen in the frontal cortex8.
To date little attention has been given to the prenatal evaluation of the developing human fetus exposed to alcohol. This is surprising given the widespread availability of diagnostic ultrasound imaging to pregnant women and the fact that alcohol exposure has been shown to produce structural abnormalities in the fetal brain which should be readily amenable to detection with ultrasound.
Only a few studies have used prenatal ultrasound imaging to evaluate human fetal brain development in women using alcohol during pregnancy. Persutte et al. noted that a heavily alcohol-exposed fetus appeared to have impaired frontal cortex growth when using a newly developed ‘frontal lobe/transcerebellar ratio’9. Wass et al.10 demonstrated that the frontal cortex and the cerebellar diameter were reduced in a population of heavily alcohol-exposed fetuses, whereas Handmaker et al.11 found reduced cerebellar growth and smaller head diameters in children prenatally exposed to alcohol. These studies suggest that a more extensive evaluation of a larger cohort of well characterized alcohol-using pregnant women could lead to the development of better ultrasound markers for FASD and support earlier identification of affected fetuses with concomitant earlier opportunities for intervention.
METHODS
A longitudinal prospective pilot study was conducted between 2004 and 2006 as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). The CIFASD is an international consortium of basic science and clinical investigations sponsored by the US National Institute of Alcohol Abuse and Alcoholism (NIAAA) and focused on addressing critical questions regarding the prenatal effects of alcohol. The pilot study was performed at two sites in Ukraine.
The study protocol was approved by an institutional review board in Ukraine and the institutional review board at the University of California, San Diego. All study participants provided written informed consent.
Recruitment of subjects and criteria for alcohol exposure
Alcohol-exposed and comparison group subjects were selected from women between 10 and 40 weeks of gestation who were screened by prenatal care providers at one of the two sites using a short standardized screening procedure. Subjects were considered eligible for the alcohol-exposed group based on quantity and frequency of alcohol consumption during pregnancy. A positive screen for quantity and frequency of alcohol consumption was defined as at least four episodes of five or more standard (as defined in the USA) drinks, at least five episodes of three to four standard drinks, or at least 10 episodes of one to two standard drinks either in the month around the time of conception or the most recent month of pregnancy. In addition, to account for women who may have denied alcohol use during pregnancy but could be considered ‘high risk’ drinkers, women were screened using two standard tools, the TWEAK (tolerance, worried, eye-opener, amnesia, cutdown) and the Alcohol Use Disorders Identification Test (AUDIT), for assessment of signs of alcohol abuse in the previous year. These standard tools have been well validated and are comprised of several short questions focused on behaviors such as tolerance to alcohol (number of drinks the individual can ‘hold’ before passing out or becoming ill), perceived annoyance of others toward the woman’s drinking, desire to cut down on drinking, history of consuming an ‘eye opener’ the morning after a drinking episode, and history of amnesia for events that happened while drinking. Points are assigned for positive responses to each of these questions. Based on standard cut-off values, a total score of ≥ 2 on the TWEAK12 or a score of ≥ 6 on the AUDIT13 was used to represent signs of risky drinking in the previous year. Women who scored above these cut-off points on one or both of the tools qualified for the exposed group regardless of whether alcohol consumption reported within pregnancy met the criteria for enrollment. Subjects were considered eligible for the comparison group based on minimal or no alcohol consumption during pregnancy as reported at the time of the initial screening, and self-reported lack of risky alcohol consuming behaviors in the previous year (i.e. both scores below the cut-off points noted above).
All eligible alcohol-exposed women were invited to enroll, and comparison group women were recruited in a 1: 1 ratio. This was accomplished following enrollment of each alcohol-exposed woman by approaching the next pregnant woman presenting for prenatal care who reported low or no alcohol exposure in response to the screening questionnaire. This was repeated sequentially until a qualified comparison woman agreed to participate. Following enrollment, all subjects participated in a 1-h interview, conducted immediately before the first study ultrasound evaluation and focused on a detailed alcohol consumption history. A timeline follow-back procedure, which has been widely used and validated in other studies of alcohol use in pregnancy14, was used to enhance the accuracy of recall. For each type of alcoholic beverage consumed each day in a 1-week period around the time of conception, and in the most recent 2 weeks in pregnancy, the number of drinks and volume were recorded. Reported quantities of each type of beverage were converted into absolute ounces of alcohol and averaged across the time frame to estimate absolute ounces of alcohol consumed per day and per drinking day.
Ultrasound examinations
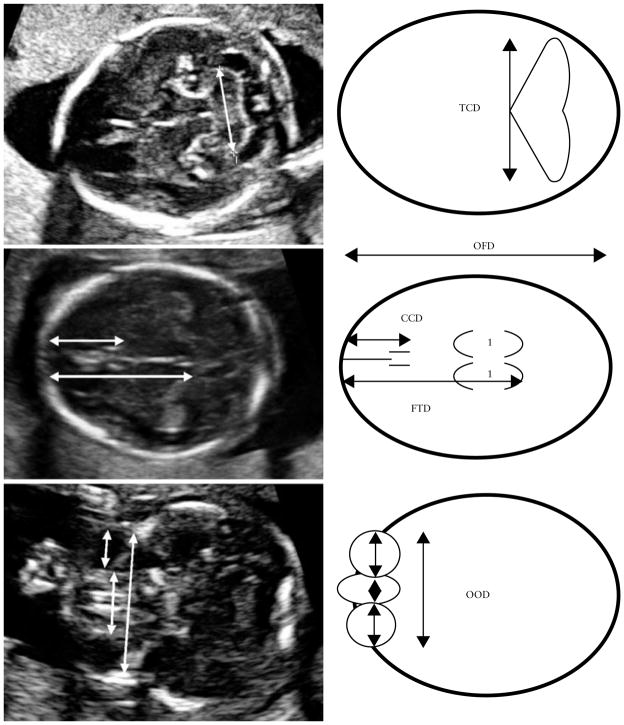
At each of the two study sites in Ukraine, pregnant women are offered several ultrasound examinations during gestation as part of their standard of care. Women were scanned routinely in the first trimester for dating, in the mid-second trimester for fetal anatomical evaluation and in the third trimester for evaluation of fetal growth. Additional specific brain growth and facial measurements were incorporated into an expanded evaluation in the second and third trimesters of pregnancy. These additional measurements included transverse cerebellar diameter (TCD) measured as the maximum diameter of the cerebellum in the standard posterior fossa view, occipitofrontal diameter, caval–calvarial distance (CCD) measured as the distance between the inner surface of the frontal calvarium and the posterior margin of the cavum septi pellucidi, frontothalamic distance (FTD) measured as the distance between the inner surface of the frontal calvarium and the posterior margin of the thalami, and orbital diameter (OD) measurements (Figure 1). One measurement was taken for each parameter.
Figure 1.
Axial ultrasound images with accompanying schematic diagrams illustrating the measured fetal brain parameters. CCD, caval–calvarial distance; FTD, frontothalamic distance; OFD, occipitofrontal diameter; OOD, outer orbital diameter; TCD, transverse cerebellar diameter.
Ultrasound measurements were performed using an Aloka SSD-650CL ultrasound machine (Aloka, Tokyo, Japan) with a convex-array transabdominal 3.5/5.0-MHz transducer. Scans were performed by certified ultrasonographers, each of whom had undergone specialized training by one of the authors of this study (A.D.H.), and who were blinded to the alcohol exposure status of the subjects at the time of each scan. All ultrasound scans were videotaped and archived for validation purposes and for future analyses. Ultrasound tapes were reviewed and measurements were verified in the USA by two of the authors of this study (A.D.H., M.K.).
Statistical analysis
Distribution of maternal demographic, lifestyle and reproductive health characteristics, and prevalence of positive AUDIT or TWEAK scores, were compared between study groups using ANOVA for continuous variables and Chi-square tests for categorical variables. Mean absolute ounces of alcohol consumed per day and per drinking day during a typical week around the time of conception and during the 2 weeks preceding the maternal interview were compared among study groups by ANOVA. Gestational age-specific percentiles of fetal growth measures, using US standards for fetal growth, were compared among alcohol-exposed and comparison subjects in univariate analysis by ANOVA and by multivariable ANOVA including adjustment for smoking. Specific fetal brain measures assessed during the second and third trimesters of pregnancy were compared among study groups by ANCOVA including adjustment for smoking and gestational age.
RESULTS
A total of 6745 women were screened at the two sites over the 2-year study period; of these a total of 166 subjects were recruited, 84 women in the alcohol-exposed and 82 in the comparison group. Not all women who enrolled and participated in the maternal interview received all of the scans involved in the study either because the woman did not return to the diagnostic center, the pregnancy ended early, or the woman enrolled later in gestation. Thus, the sample sizes for second-trimester scans include 66 women in the alcohol-exposed and 64 women in the comparison group, and the sample sizes for the third-trimester scans were reduced to 47 and 31 subjects, respectively.
The first maternal interview was conducted on average between 18 and 19 weeks’ gestation. Table 1 describes maternal demographic, lifestyle and reproductive health characteristics by alcohol exposure group. Women in the alcohol-exposed group were more likely to be single mothers from lower socioeconomic groups, to have unplanned pregnancies and to be current smokers, relative to the comparison group.
Table 1.
Maternal demographic, lifestyle and reproductive health characteristics according to alcohol exposure status
| Characteristic | Alcohol exposed (n = 84) | Comparison (n = 82) | P |
|---|---|---|---|
| Unmarried | 10.7 | 1.2 | 0.02 |
| Socioeconomic status below average* | 51.2 | 31.7 | < 0.01 |
| Unplanned current pregnancy | 57.1 | 35.4 | < 0.01 |
| Primigravid | 36.9 | 46.3 | NS |
| Nulliparous | 60.0 | 62.0 | NS |
| Any previous miscarriage(s) | 25.0 | 20.9 | NS |
| Previous pregnancy termination(s) | 63.6 | 62.8 | NS |
| Smoker | < 0.01 | ||
| Never been | 49.4 | 97.5 | |
| Past, quit before pregnancy | 8.4 | 0 | |
| Past, quit after realized pregnant | 24.1 | 2.5 | |
| Current | 18.1 | 0 | |
| Maternal age (years) | 26.2 ± 5.7 | 24.7 ± 4.1 | NS |
| GA at interview (weeks) | 18.2 ± 7.8 | 19.2 ± 6.5 | NS |
Values are % or mean ± SD.
Based on Hollingshead categories 1–5 derived from occupation and education of mother and father, with 1 being the highest; below average socioeconomic status is defined as Hollingshead category 4 or 5. GA, gestational age; NS, not significant.
No woman who was positive on TWEAK or AUDIT screening maintained that they drank little or no alcohol during pregnancy. Table 2 describes the characteristics of maternal alcohol use before and during pregnancy. Consistent with the group selection criteria, indicators of alcohol abuse, as reported over the previous 12 months, were significantly more common in the alcohol-exposed vs. comparison women. Similarly, average absolute ounces of alcohol consumed per day during both time periods was significantly greater in the alcohol-exposed group than in comparison women. Absolute ounces of alcohol per drinking day also varied by group, but the means did not differ significantly owing to the very small number of women in the comparison group who reported any alcohol use at all (most women in the comparison group had no drinking days). The subset of subjects in each group who participated in the third-trimester scan did not differ materially in any of the demographic or lifestyle characteristics that were measured from those who participated in the second-trimester scan (data not shown).
Table 2.
Characteristics of maternal alcohol use in alcohol-exposed and comparison pregnancies
| Signs of abuse | Alcohol exposed (n = 84) | Comparison (n = 82) | P |
|---|---|---|---|
| Past 12 months | |||
| Tolerance ≥ 6 (‘hold’ version)* | 67.1 | 1.4 | < 0.001 |
| AUDIT ≥ 6 | 27.4 | 0 | < 0.001 |
| TWEAK ≥ 2 | 69.6 | 1.4 | < 0.001 |
| Periconceptional period | |||
| AA per day | 1.07 ± 1.4 | 0.02 ± 0.2 | < 0.001 |
| AA per drinking day | 5.01 ± 4.6 | 2.96 ± 4.8† | NS |
| Pregnancy–last 2 weeks | |||
| AA per day | 0.14 ± 0.3 | 0.0004 ± 0.002 | < 0.001 |
| AA per drinking day | 2.23 ± 3.6 | 0.20 ± 0.000† | NS |
Values are % or mean ± SD.
Tolerance ≥ 6 defined as self-reported ability to consume six or more standard drinks on an occasion before passing out or feeling too ill to continue.
Based on four women in the comparison group who reported any alcohol consumption (i.e. had any drinking day) in the periconceptional period and two women in the comparison group who reported any alcohol consumption in the most recent 2 weeks of pregnancy. AA, absolute ounces of alcohol; AUDIT, Alcohol Use Disorders Identification Test; NS, not significant; TWEAK, tolerance, worried, eye-opener, amnesia, cutdown.
Second-trimester ultrasound examination
Standard biometric and additional study-specific ultrasound measurements were compared between the exposure groups (66 alcohol-exposed and 64 comparison women). Unadjusted analysis of second-trimester ultrasound biometry showed significant differences between groups only for femur length percentile (P = 0.004); this difference remained significant after control for maternal smoking (P = 0.025) (Table 3). For the specific brain measurements, after adjustment for gestational age, only CCD was significantly shorter in alcohol-exposed fetuses (P < 0.05). After additional adjustment for maternal smoking, both CCD and FTD were significantly decreased in the alcohol-exposed group relative to the comparison group (P < 0.05) (Table 4).
Table 3.
Fetal biometric growth parameters, expressed as gestational age-specific percentiles, assessed during the second trimester in alcohol-exposed and comparison pregnancies
| Parameter | Alcohol exposed (n = 66)* | Comparison (n = 64)* | P (unadjusted) | P (adjusted)† |
|---|---|---|---|---|
| Estimated fetal weight | 31.8 ± 16.5 | 34.2 ± 16.1 | NS | NS |
| Biparietal diameter | 53.9 ± 28.1 | 54.0 ± 27.1 | NS | NS |
| Head circumference | 37.1 ± 20.1 | 40.1 ± 17.0 | NS | NS |
| Abdominal circumference | 36.0 ± 28.6 | 43.1 ± 32.7 | NS | NS |
| Femur length | 52.6 ± 25.2 | 64.5 ± 20.8 | 0.004 | 0.025 |
Values are mean ± SD percentiles.
Sample size might vary owing to missing values.
Adjusted for smoking (never/past/sometime during pregnancy). NS, not significant.
Table 4.
Fetal brain measures assessed during the second trimester in alcohol-exposed and comparison pregnancies
| Parameter | Alcohol exposed (n = 66)* | Comparison (n = 64)* | P† | Smoking-adjusted P‡ |
|---|---|---|---|---|
| Transverse cerebellar diameter (mm) | 21.8 ± 0.2 | 22.0 ± 0.2 | NS | NS |
| Occipitofrontal diameter (mm) | 68.5 ± 0.6 | 68.3 ± 0.6 | NS | NS |
| Caval–calvarial distance (mm) | 25.6 ± 0.3 | 26.6 ± 0.4 | < 0.05 | < 0.05 |
| Frontothalamic distance (mm) | 40.5 ± 0.5 | 41.6 ± 0.5 | NS | < 0.05 |
| Outer orbital diameter (mm) | 36.1 ± 0.3 | 35.9 ± 0.9 | NS | NS |
| Interorbital distance (mm) | 11.0 ± 0.2 | 11.3 ± 0.2 | NS | NS |
| Orbital diameter (mm) | 11.5 ± 0.2 | 11.6 ± 0.2 | NS | NS |
Values are mean ± SE.
Sample size might vary owing to missing values.
Adjusted for gestational age.
Adjusted for gestational age and smoking (never/past/sometime during pregnancy). NS, not significant.
Third-trimester ultrasound examination
Data were available for 47 alcohol-exposed and 31 comparison pregnancies for third-trimester ultrasound measures. Standard biometric measures were significantly different between groups only for biparietal diameter percentile measurement (P < 0.05); this difference persisted after control for maternal smoking (P < 0.05) (Table 5). FTDs were significantly shorter in alcohol-exposed fetuses after adjustment for gestational age (P < 0.01). Consistent with the findings in the second trimester, this difference persisted after controlling for maternal smoking (P < 0.05) (Table 6). In contrast, CCDs were similar in the two groups in the third trimester; the difference observed in the second-trimester comparison was not seen in the third trimester. OD was significantly shorter in alcohol-exposed fetuses relative to the comparison group after adjustment for gestational age and control for maternal smoking (P < 0.05) (Table 6).
Table 5.
Fetal biometric growth parameters, expressed as gestational age-specific percentiles, assessed during the third trimester in alcohol-exposed and comparison pregnancies
| Parameter | Alcohol exposed (n = 47)* | Comparison (n = 31)* | P (unadjusted) | P (adjusted)† |
|---|---|---|---|---|
| Estimated fetal weight | 30.3 ± 16.4 | 32.9 ± 17.6 | NS | NS |
| Biparietal diameter | 53.6 ± 30.2 | 69.8 ± 27.6 | < 0.05 | < 0.05 |
| Head circumference | 44.5 ± 25.4 | 50.3 ± 22.9 | NS | NS |
| Abdominal circumference | 46.8 ± 26.5 | 56.3 ± 24.2 | NS | NS |
| Femur length | 47.1 ± 27.8 | 45.5 ± 24.9 | NS | NS |
Values are mean ± SD percentiles.
Sample size might vary owing to missing values.
Adjusted for smoking (never/past/sometime during pregnancy). NS, not significant.
Table 6.
Fetal brain measures assessed during the third trimester in alcohol-exposed and comparison pregnancies
| Parameter | Alcohol exposed (n = 47)* | Comparison (n = 31)* | P† | Smoking-adjusted P‡ |
|---|---|---|---|---|
| Transverse cerebellar diameter (mm) | 41.6 ± 0.4 | 40.9 ± 0.5 | NS | NS |
| Occipitofrontal diameter (mm) | 106.7 ± 1.4 | 107.5 ± 1.8 | NS | NS |
| Caval–calvarial distance (mm) | 42.3 ± 0.6 | 43.4 ± 0.7 | NS | NS |
| Frontothalamic distance (mm) | 64.2 ± 0.7 | 67.2 ± 0.8 | < 0.01 | < 0.05 |
| Outer orbital diameter (mm) | 54.6 ± 0.6 | 54.7 ± 0.7 | NS | NS |
| Interorbital distance (mm) | 15.6 ± 0.4 | 14.8 ± 0.4 | NS | NS |
| Orbital diameter (mm) | 16.2 ± 0.3 | 17.2 ± 0.3 | < 0.05 | < 0.05 |
Values are mean ± SE.
Sample size might vary owing to missing values.
Adjusted for gestational age.
Adjusted for gestational age and smoking (never/past/sometime during pregnancy). NS, not significant.
DISCUSSION
This pilot study suggests that, among women who report moderate-to-heavy amounts of alcohol use in pregnancy, a number of measurements on routine prenatal ultrasound imaging in the second and third trimesters of pregnancy may be markers for an alcohol-affected fetus, although a follow-on study with a larger sample size will be necessary to evaluate the clinical utility of these markers. Shortened femur length in the exposed group relative to the comparison group, as measured by second-trimester ultrasound examination, is consistent with the well established incidence of growth deficiency including shorter birth length in infants with prenatal alcohol exposure15. Of particular interest, both the CCD and the FTD were significantly shorter on the second-trimester ultrasound examination in fetuses prenatally exposed to alcohol relative to the comparison group. This mean difference continued to be true for FTD as measured in the third trimester. This is an exciting finding suggesting that, with further study, it may be possible to demonstrate that clinically relevant impaired growth of the frontal cortex is detectable as early as 24 weeks in these alcohol-exposed fetuses.
These findings are consistent with those of Wass et al.10, who evaluated a multiethnic group of 70 women who consumed moderate-to-large amounts of alcohol at the time of conception in comparison with 97 women who reported consuming little or no alcohol at the time of conception. Subjects received one to six study-related ultrasound examinations depending on timing of study entry, including four brain measurements: TCD, FTD, CCD and biparietal diameter. FTD and CCD were the only two brain measures associated with maternal alcohol use. The authors concluded that there was evidence of a disproportionate effect of alcohol on the frontal cortex rather than a global effect on the developing brain.
The findings of the present study, as well as those of Wass et al.10, are less consistent with results reported by Handmaker et al.11 who studied 167 multiethnic pregnant women with risky drinking reported at conception. Nearly half of the subjects reported discontinuation of alcohol after pregnancy recognition. Measures from a single ultrasound examination performed at some point between 18 and 42 weeks’ gestation were abstracted from medical records. Brain measurements including TCD, lateral ventricular atrial diameter and diameter of the cisterna magna were collected as well as standard biometric measurements. Any alcohol consumption among heavy drinkers following pregnancy recognition was significantly associated with reduced transcerebellar diameter, but not with standard fetal biometric growth parameters.
The findings of the present study are compatible with a mouse model of FAS reported by Sulik et al.16. This group noted that maternal alcohol exposure at stages of embryogenesis corresponding to week 3 of human embryonic development resulted in CNS abnormalities that involve the forebrain, including hypoplasia or aplasia of the corpus callosum and septal nuclei, hypoplasia of the basal ganglia and deficiency in the hippocampus and the anterior cingulate cortex.
We are not aware of any previous human study that has evaluated OD through prenatal ultrasound examination as a potential marker for alcohol-affected fetuses. The fact that shorter OD was associated with alcohol exposure in this study suggests that this measurement could represent a prenatal marker for shortened palpebral fissure length, one of the cardinal craniofacial features of FAS.
This pilot study has some limitations. Although specific quantity, frequency and pattern of alcohol use was collected from study participants, the sample size was not sufficient for separate analysis of women who continued to drink throughout pregnancy at varying amounts. There could also have been bias in the characteristics that were measured in the women who returned for second- or third-trimester ultrasound examinations compared with those who did not. This, along with the reduced sample size for later scans, may have had an impact on the differences in findings among women who received the second-trimester ultrasound examination vs. those who were scanned in the third trimester. We did not adjust for multiple testing in the analysis of the pilot study data; therefore, some of the observed differences in findings between the second and third trimesters could be due to chance. In addition, the use of maternal report as the sole measure of alcohol use may under-represent true alcohol consumption levels. However, the prospective design of the study and the standard methods for collecting alcohol consumption data using validated timeline follow-back procedures may have enhanced accurate recall and reporting. Other strengths of this study include the standardized and repeated ultrasound measures, using techniques that are readily translatable to the clinical setting.
Results from the pilot phase of this study suggest that further work is warranted and that the implications of our findings could be important from a public health perspective. The data support the notion that earlier intervention with alcohol-affected children can lead to improved outcomes. Therefore, identification of affected children in prenatal life could lead to the initiation of such interventions at the earliest possible time. From the standpoint of the alcohol-consuming mother who may continue drinking throughout pregnancy, it is important to recognize that the developing fetal brain continues to be affected by maternal alcohol consumption throughout the second and third trimesters. Therefore, information provided by prenatal ultrasound examination may affect drinking behavior during the remaining weeks of that pregnancy.
In summary, at the present time these pilot data do not provide clinically relevant standards for measurements on prenatal ultrasound examination that can support prenatal diagnosis of alcohol-affected fetuses. However, they represent a promising step forward towards earlier recognition of pregnancies likely to result in a child with FASD. Further analyses currently being conducted through the CIFASD consortium will examine the correlation between these prenatal ultrasound measures and alcohol-related physical features identified in newborns. Additional studies are needed to describe the predictive value of these same prenatal ultrasound measures for neurobehavioral impairment.
Acknowledgments
This study was funded by National Institutes of Health–NIAAA grant (number 5 U24 AA014811).
References
- 1.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 2.Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- 3.McGee CL, Riley EP. Brain imaging and fetal alcohol spectrum disorders. Ann Ist Super Sanita. 2006;42:46–52. [PubMed] [Google Scholar]
- 4.Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 5.Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 6.Clarren SK, Alvord EC, Jr, Sumi SM, Streissguth AP, Smith DW. Brain malformations related to prenatal exposure to ethanol. J Pediatr. 1978;92:64–67. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- 7.Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I–V. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 8.Riley EP, McGee CL, Sowell ER. Teratogenic effects of alcohol: a decade of brain imaging. Am J Med Genet C Semin Med Genet. 2004;127:35–41. doi: 10.1002/ajmg.c.30014. [DOI] [PubMed] [Google Scholar]
- 9.Persutte WH, Coury A, Hobbins JC. Correlation of fetal frontal lobe and transcerebellar diameter measurements: the utility of a new prenatal sonographic technique. Ultrasound Obstet Gynecol. 1997;10:94–97. doi: 10.1046/j.1469-0705.1997.10020094.x. [DOI] [PubMed] [Google Scholar]
- 10.Wass TS, Persutte WH, Hobbins JC. The impact of prenatal alcohol exposure on frontal cortex development in utero. Am J Obstet Gynecol. 2001;185:737–742. doi: 10.1067/mob.2001.117656. [DOI] [PubMed] [Google Scholar]
- 11.Handmaker NS, Rayburn WF, Meng C, Bell JB, Rayburn BB, Rappaport VJ. Impact of alcohol exposure after pregnancy recognition on ultrasonographic fetal growth measures. Alcohol Clin Exp Res. 2006;30:892–898. doi: 10.1111/j.1530-0277.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- 12.Russell M, Martier SS, Sokol RJ, Mudar P, Bottoms S, Jacobson S, Jacobson J. Screening for pregnancy risk-drinking. Alcohol Clin Exp Res. 1994;18:1156–1161. doi: 10.1111/j.1530-0277.1994.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 13.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 14.Sokol RJ, Martier S, Ernhart C. Identification of alcohol abuse in the prenatal clinic. In: Chang NC, Chao M, editors. Early Identification of Alcohol Abuse. Alcohol, Drug Abuse, and Mental Health Administration Research; Rockville, MD: 1983. monograph number 17. [Google Scholar]
- 15.Lumeng JC, Cabral HJ, Gannon K, Heeren T, Frank DA. Prenatal exposures to cocaine and alcohol and physical growth patterns to age 8 years. Neurotoxicol Teratol. 2007;29:446–457. doi: 10.1016/j.ntt.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sulik KK. Critical periods for alcohol teratogenesis in mice, with special reference to the gastrulation stage of embryogenesis. Ciba Found Symp. 1984;105:124–141. doi: 10.1002/9780470720868.ch8. [DOI] [PubMed] [Google Scholar]