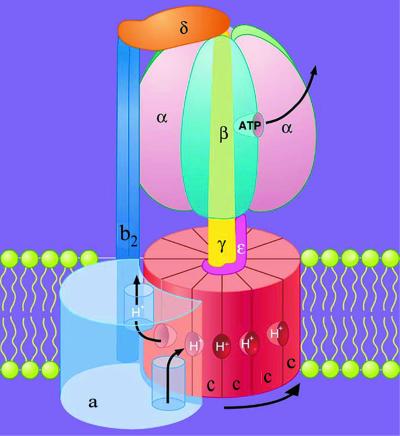
Figure 1.
Model for the structure and mechanism of E. coli FOF1. Adapted from Duncan et al. (24). The membrane-embedded FO sector catalyzes proton transport and consists of one copy of subunit a, a dimer of b subunits, and an oligomeric ring of c subunits. The hydrophilic F1 sector extends ≈120 Å from the cytoplasmic face of the membrane and contains five subunits with the stoichiometry α3β3γ1δ1ɛ1. The α and β subunits alternate in a hexamer that surrounds a central asymmetric core consisting of the γ subunit. Three catalytic nucleotide-binding sites (one visible) are located at alternate α/β subunit interfaces, primarily on the β subunits. F1 binds to FO through two distinct linkages: (i) a central stalk (or rotor) comprised of γɛ and anchored to the c ring, and (ii) a peripheral stalk (or stator), comprised of b2δ, that anchors α3β3 to the a subunit. Two partial channels for proton transport, each accessible to a different side of the membrane, are thought to be located between the a and c subunits. During ATP synthesis, each proton would enter through the periplasmic channel and bind to a deprotonated c subunit. The c ring then would rotate one step to the right relative to subunit a, and net transport would occur as a previously protonated c subunit moves into alignment with the cytoplasmic channel and loses its proton. Because the c ring is anchored to γɛ and subunit a is tethered to α3β3 by b2δ, rotation of the c ring relative to subunit a forces γ to rotate relative to the catalytic subunits, thus driving the binding changes required to achieve net synthesis of ATP.