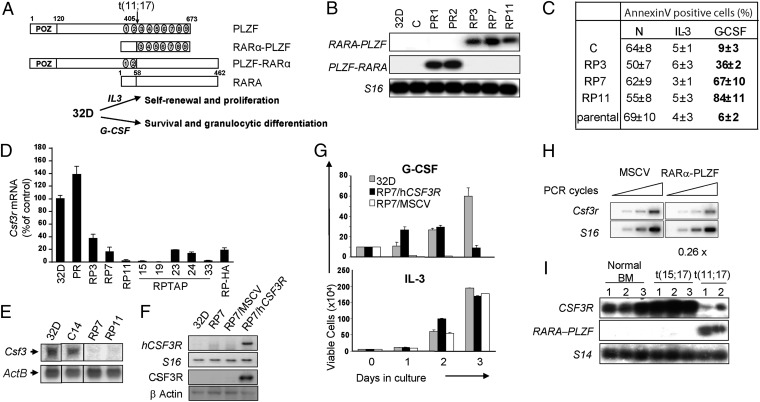
Fig. 1.
RARα-PLZF impairs Csf3r expression and cell survival in response to G-CSF. (A) Schematic representation of PLZF, RARα, and the reciprocal fusion proteins of the t(11;17) translocation, RARα-PLZF, and PLZF-RARα. (B) Confirmation of ectopic PLZF-RARα or RARα-PLZF expression in 32D clones. S16 is shown as a control for variations in input RNA loading. (C) Flow cytometry analysis of 32D clones in the presence or absence of IL-3 or G-CSF for 24 h. Apoptotic cells were labeled with Annexin V, and dead cells were excluded by propidium iodide staining. Shown are the average ± SD of two to five independent experiments. (D) Csf3r mRNA levels in RARα-PLZF expressing clones (RP7, RP11, and RP-TAP) and in polyclonal PLZF-RARα expressing cells grown in IL-3 medium. Csf3r mRNA levels were determined by real-time PCR analysis, normalized to Hprt levels and calculated as percent of 32D parental cells. Data shown are the average ± SD of two independent experiments performed in quadruplates. (E) Northern blot analysis of Csf3r in 32D parental cells, control cells (C), and RARα-PLZF expressing clones 7 and 11. ActB is shown as a loading control. (F) Confirmation of Csf3r expression in RP7 transfected cells. RP7 cells were either transduced with the huCSF3R expression vector (RP7/huCSF3R) or the empty MSCV vector (RP7/MSCV). Expression levels of huCSF3R mRNA were measured by semiquantitative RT-PCR (Upper) and of CSF3R protein were measured by Western blotting (Lower). S16 is shown as a control for input RNA and β-actin, for input protein. (G) huCSF3R expression rescues G-CSF–induced cell survival in RP7 cells up to 48 h but fails to provide long-term cell survival by G-CSF. Cells were grown in the presence of G-CSF or IL-3, and viable cells were evaluated by trypan blue exclusion (average ± SD, n = 2 in triplicates). (H) Csfr3 expression in primary fetal liver cells expressing RARα-PLZF. Primary fetal liver cells from 12.5 dpc embryos were transduced with the empty vector or RARα-PLZF. Total RNA extracted from CD11b+GFP+ cells were analyzed by semiquantitative RT-PCR (n = 2). (I) CSF3R mRNA levels are decreased in leukemic cells from APL patients with t(11;17) translocation. Total bone marrow cells from three normal donors and three t(15;17) and two t(11;17) APL patient samples were analyzed by RT-PCR for the presence of CSF3R transcripts. S14 is shown as a control for input RNA.