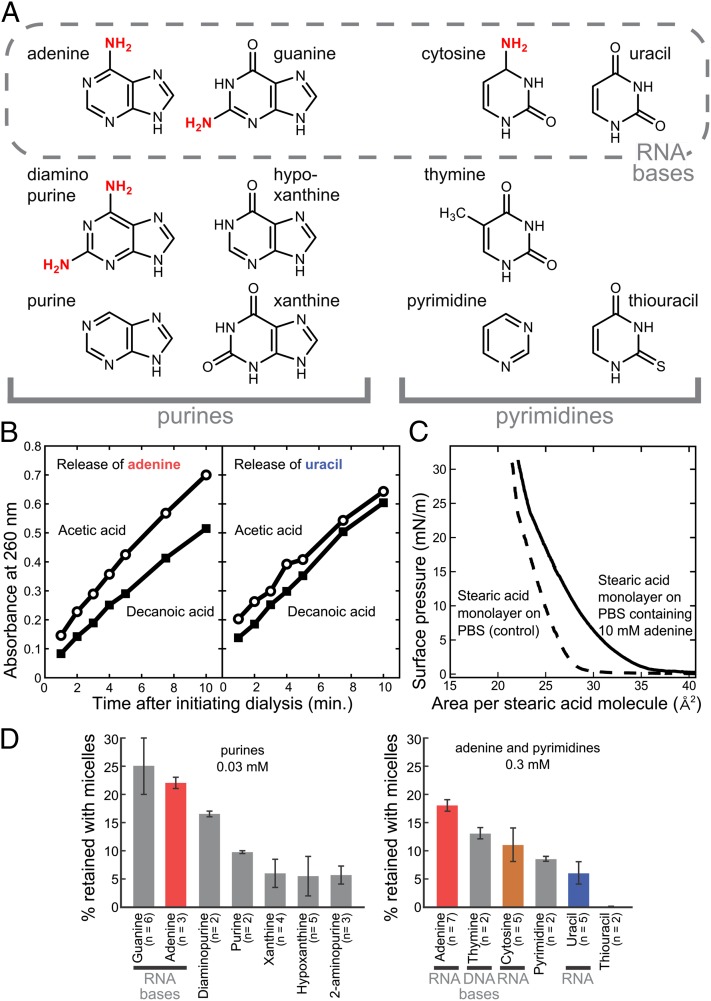
Fig. 1.
Decanoic acid aggregates selectively bind heterocyclic nitrogenous bases. (A) Structures of purines and pyrimidines tested for interactions with decanoic acid aggregates. Diaminopurine contains an amine at the 2-position in addition to the 6-position as in adenine; 2-aminopurine, also tested in some experiments, has an amine only at the 2-position. Amine substituents are indicated in red. (B) Adenine dialyzes more slowly from a decanoic acid solution than from an acetic acid solution. (Left) Results of a representative experiment in which adenine, at 15 mM, diffused from either 180 mM decanoic acid or from 180 mM acetic acid. Aliquots of dialysis buffer were collected at indicated times and assayed for adenine by measuring absorbance at 260 nm. The rate of release was 24 ± 5% lower from decanoic acid (P < 0.05). (Right) Results of a corresponding representative control experiment with uracil in place of adenine. The rate of release was 8 ± 7% greater, not lower, from decanoic acid (P > 0.05). (C) The presence of 10 mM adenine in a subphase of PBS increases the surface pressure of a Langmuir monolayer of stearic acid. Measurement uncertainty is ±1 mN/m. Stearic acid (18 carbons) was used instead of decanoic acid because the latter does not form a stable Langmuir monolayer. (D) Nucleobases are retained with decanoic acid micelles during ultrafiltration. A solution of 180 mM decanoic acid and each base at 0.03 mM (for purines) or 0.3 mM (for pyrimidines) was partially centrifuged through a 3-kDa–cutoff filter. These concentrations optimize both the percentage of base retained by micelles and the detection of base by absorbance; adenine was evaluated at both 0.3 and 0.03 mM to enable comparison of all the bases. Values are averages, and error bars represent average deviations. (The difference between the means for cytosine and uracil is significant based on Student t test: P = 0.028 by a one-tailed test and 0.056 by a two-tailed test.)